|
# # # # Neurotrophic factors – like Glial cell line-derived neurotrophic factor (or GDNF) – hold great hope for regenerative therapy in Parkinson’s research. New research, however, indicates that simply injecting the protein into the brain may not be enough. Scientists at Rush University Medical Center (in Chicago) conducted a postmortem analysis of brains from people who passed away with Parkinson’s and made an intriguing discovery. They found that many of the remaining dopamine neurons appear to not be producing a protein called Ret, which is required for GDNF signaling. In addition, other components of GDNF signaling pathway were missing. In today’s post, we will review the background of this new study, outline what the study found, and discuss the implications of the research. # # # # |
GDNF. Source: Wikipedia
Glial cell line-derived neurotrophic factor (or GDNF) is a topic that gets a lot of reader attention on the SoPD. It is a tiny protein that holds great hope for the Parkinson’s community in terms of providing a potential neuroprotective and regenerative therapy.
GDNF is a type of neurotrophic factor, which are small naturally-occurring proteins that nurture neurons and support their growth. There are different kinds of neurotrophic factors, and the testing of some of them in preclinical models of Parkinson’s has generated encouraging results (particularly in the case of GDNF – click here to read a previous SoPD post on this topic).
But the translation of those initial results in cell culture and animal models of Parkinson’s has been difficult in clinical trials of neurotrophic factors.
This has led to many questions being asked within the research community about the nature of biological signaling pathways involved with neurotrophic factors and whether they might be affected in Parkinson’s.
The majority of the neurotrophic factors that have been tested in models of Parkinson’s and in clinical trials for Parkinson’s belong to a branch that requires the RET signaling pathway to be available to have their neuroprotective effect.
What is the RET signaling pathway?
Ret proto-oncogene (or Ret) is a receptor of certain neurotrophic factors, like GDNF.
What is a receptor?
On the outer surface of cells there are small proteins called receptors, which act like switches for certain biological processes. Receptors will wait for another protein (referred to as a ligand) to come along and activate them. Activation of a receptor results in biological pathways inside the cell being turned on, resulting in an intracellular response.

When GDNF protein floating around outside of a cell binds to and activates RET, it activates a vast cascade inside the cell that can result in neuroprotective effects.

But given the lack of positive results coming from clinical trials exploring GDNF (and associated neurotrophic factors like neurturin – click here to read a previous SoPD post on this topic), researchers have been questioning whether the Ret signaling pathway is functioning normally in Parkinson’s.
|
# RECAP #1: Neurotrophic factors are molecules that stimulate growth and survival in neurons. Research in models of Parkinson’s have suggested that they could be potentially useful as a therapy. Translation of that preclinical research to humans, however, has been complicated. And now questions are being asked about whether the biological pathways involved with these molecules are functioning properly in the PD brain. # |
What evidence is there that something might be wrong?
In 2012, a research report was published which indicated that high levels of the Parkinson’s-associated alpha synuclein protein resulted in the reduction of Ret protein in dopamine neurons.
Here is that report:
Title: α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons.
Authors: Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Björklund A.
Journal: Sci Transl Med. 2012 Dec 5;4(163):163ra156.
PMID: 23220632 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that GDNF could not protect dopamine neurons in an alpha synuclein model of Parkinson’s (and this result was supported by the findings of an independent research group – click here to read more about that). The researchers found that GDNF was unable to have its effect because the high levels of alpha synuclein were reducing the levels of Ret.
Remind me again, what alpha synuclein is?
Alpha synuclein is one of the most common proteins in the brain (making up about 1% of the protein in neurons). The exact function of alpha synuclein is not well understood, but research suggests that it plays a role in multiple cellular functions – including being involved in neurotransmitter release.
But in Parkinson’s, something changes.
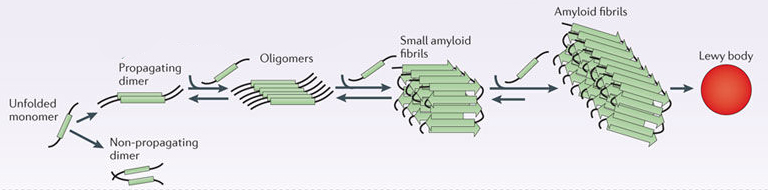
For some reason, in many cases of Parkinson’s alpha synuclein protein starts to cluster and clump together. And this “aggregated” form of alpha synuclein is believed to become toxic, messing with the normal functioning of cells.
The findings of the 2012 study suggested that alpha synuclein might be messing with the normal functioning of the Ret signaling pathway and this naturally raised questions as to whether GDNF would work as a therapeutic in the brains of people with Parkinson’s. If high levels of alpha synuclein reduces levels of Ret protein, then will GDNF be able to have any neuroprotective function in the Parkinsonian brain?
It is important to remember that the 2012 paper was largely preclinical experiments, but recently, researchers at Rush University in Chicago have conducted a more in-depth postmortem analysis of sections of brain tissue from people who passed away with Parkinson’s to address investigate this matter.
Here is their report:
Title: GDNF signaling in subjects with minimal motor deficits and Parkinson’s disease.
Authors: Chu Y, Kordower JH.
Journal: Neurobiol Dis. 2021 Mar 5:105298. Online ahead of print.
PMID: 33684514 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers collected brain tissue from older adults who passed away with no clinical motor deficit (the “control” group; N=6), minimal motor deficits (N=10), or a clinical & pathologic diagnosis of Parkinson’s (N=10).
There was no loss of dopamine neurons in the substantia nigra in the control brains, while minimal motor deficit individuals displayed mild or moderate loss of dopamine neurons and the PD cases exhibited moderate or severe dopamine neuron loss.
What is the substantia nigra?
By the time a person is presenting the motor features characteristic of Parkinson’s (slowness of movement, rigidity, and a resting tremor) and they are being referred to a neurologist for evaluation, they will have lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain.
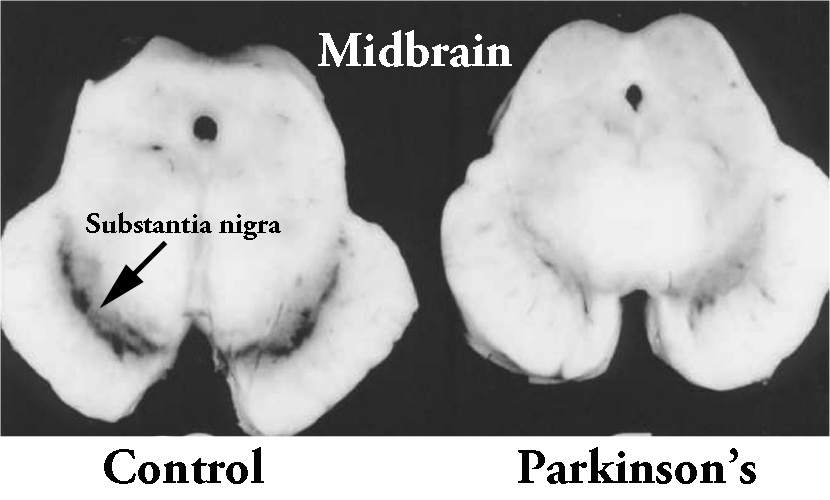
The dopamine neurons reside in a region of the midbrain, called the substantia nigra. The substantia nigra is normally visible by eye because the dopamine neurons produce a dark pigment (called neuromelanin). As the image above illustrates, people with Parkinson’s have less of this pigmentation as a result of the lost dopamine neurons.
When the researchers analysed the three groups of brains for Ret protein, they found that in the control group, virtually all of dopamine neurons displayed RET protein. In the mild motor group (MMD in the image below), they found that Ret protein levels were only “slightly reduced relative” to the control group (see panels C & D in the image below) – 98% of the remaining dopamine neurons in the mild motor group had Ret protein.
But when the investigators examined Ret protein in the Parkinson’s brains, they found a lot of dopamine neurons (distinguished by neuromelanin – the brown spots in panel F below) that did not have Ret protein (black staining in panel F). Approximately 15% of the remaining dopamine neurons had no detectable levels of Ret protein.
Ret levels in the dopamine neurons. Source: Sciencedirect
Due to the overall loss of dopamine neurons in the Parkinsonian brains, the overall levels of Ret protein in the midbrain were only 1/3 of that observed in the control group.
Interesting. Was that all that they saw?
No, the researchers also looked at ‘downstream’ proteins involved in the Ret signaling pathway to see if it was just Ret that was reduced.
Specifically, the investigators analysed levels of phosphorylated ribosomal protein S6, which is a protein involved in the Ret signaling pathway and an indicator of whether upstream activation of the neuroprotective pathways. After GDNF activates Ret, phosphorylated ribosomal protein S6 is typically activated.
Like Ret, the researchers found that there were good levels of phosphorylated ribosomal protein S6 in the dopamine neurons of the control group (NMD in the image below), suggesting that GDNF signaling pathway is functioning normal in aging. But the number of dopamine neurons with detectable levels of phosphorylated ribosomal protein S6 in the mild motor group (MMD) were significantly lower, and further reduced in the Parkinson’s group.

But what percentage neurons with Ret have alpha synuclein aggregates? Was the reduced levels of Ret protein due to alpha synuclein?
This is a difficult question to answer, but the investigators did try by looking at how many neurons in the substantia nigra with and without alpha synuclein aggregates had Ret protein. They found that alpha synuclein inclusions in neurons was associated with a large reduction in Ret protein:

And the investigators didn’t stop there.
Next, they analysed the brains of two primates that had been injected in the midbrain with a virus that causes the production of high levels of alpha synuclein. They found reduced levels of Ret and phosphorylated ribosomal protein S6 in the remaining dopamine neurons of these animals.
The investigators concluded their study by writing that their results “indicate that for neurotrophic factors to be effective in patients with minimal motor deficits or Parkinson’s, these factors would likely have to upregulate [read: boost levels of] RET and phosphorylated ri–bosomal protein S6″ in the remaining dopamine neurons in substantia nigra.
|
# # RECAP #2: Preclinical data indicated that high levels of alpha synuclein were associated with reduced levels of the GDNF receptor Ret. Recent post-mortem analysis in human brains from people who passed away with Parkinson’s found reduced levels of Ret and other GDNF pathway associated proteins, suggesting a need to increase their activity in order for GDNF to have an effect. # # |
So what does this mean for GDNF research?
Well, firstly it would be useful to see an independent replication of these results in a larger collection of samples. This has been an area of debate for a while, so further data would be useful.
It should also be noted that in the Decressac report mentioned above, the scientists were able to rescue the reduced levels of Ret effect by boosting levels of a protein called Nurr1.
What is Nurr1?
Nurr1 is a transcription factor – a protein that is involved in the process of converting (or transcribing) DNA into RNA.

In the case of Nurr1, it is a very important transcription factor for activating a lot of genes (including Ret) that are required for making and maintaining a dopamine neuron (Click here to read a previous SoPD post about Nurr1).
In the Decressac report, they found that enhancing Nurr1 in dopamine neurons was able to increase Ret levels, which resulted in a restoration of the neuroprotective effect of GDNF in the alpha synuclein model of Parkinson’s.
How can we boost Nurr1 levels?
There has been a great deal of research over the last 20 years looking for Nurr1 activators. Biotech companies have come and gone in the effort. It’s been one of those “holy grail” endeavours.
A number of molecules have been proposed as Nurr1 activator, but a recent report exploring the nature of many of them suggests that they are not ideal for the task.
Here is the report:

Authors: Munoz-Tello P, Lin H, Khan P, de Vera IMS, Kamenecka TM, Kojetin DJ.
Journal: J Med Chem. 2020 Dec 24;63(24):15639-15654.
PMID: 33289551
In this study, the researchers investigated the potential of 12 Nurr1 activators that have been previously reported. They found that most of the reported Nurr1 activators do not appear to function by direct binding to Nurr1 protein. Only the malaria drugs amodiaquine and chloroquine, plus the experimental molecule cytosporone B were found to directly bind to Nurr1, but their ability to activate Nurr1 appears to be cell-type specific (in some cells these molecules had no effect on Nurr1-related transcription). In addition, amodiaquine and chloroquine have side effects that do not make them ideal for long term use.
The researchers concluded that their results may help “influence medicinal chemistry effort“ to design better Nurr1 activators, but that improved Nurr1 activators would be required.
Great, so where does that leave us with neurotrophic factors?
An alternative approach to focusing on GDNF is to shift our attention to novel targets, particularly ones that do not necessarily involve Ret.
Such as?
In a recent SoPD post we discussed a couple of examples: Growth differentiation factor 5 (or GDF5) and a BMP5/7 combo (Click here to read that post).

More research on novel neurotrophic approaches for Parkinson’s may highlight a more promising means of achieving the long-awaited potential of neuroprotective/regenerative therapy.
So what does it all mean?
As I mentioned there has been a rather animated intellectual debate in a certain corner of the Parkinson’s research community about whether the pathways underlying GDNF signaling are functioning properly in the Parkinsonian brain. This question has been proposed as one of the possible reasons why the translation of amazing preclinical results has been so difficult. An initial open label pilot clinical study indicated great promise, but larger blinded studies have led to a roller coast saga that has impacted many.
New research provides evidence that the GDNF signaling pathway may not be completely intact in Parkinson’s, which brings into question the potential utility of this experimental therapy. Corrective solutions have been proposed (enhancing Nurr1 activity), but significant challengers still stand in the way of this approach (finding a Nurr1 activator).
As mentioned above, a larger, independent replication and expansion of the research report reviewed in today’s post is required. It would be interesting to explore what is happening to the Ret signaling pathway in sub-types of Parkinson’s where alpha synuclein does not appear to be playing a significant role (such as Parkin- or PINK1-associated Parkinson’s). Perhaps there is a specific cohort in the PD community better suited for GDNF as a potential therapy.
In every problem there is opportunity, however, and if the Ret signaling pathway is found to be weakened in many cases of PD, perhaps the opportunity here is for the research community to broaden the scope of neurotrophic factors being evaluated in PD. Maybe a neurotrophic factor that does not depend on the Ret signaling pathway can help to acheived the dream of regenerative research.
It is certain worth exploring.
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Nature