|
# # # # A lot of Parkinson’s research has focused on a neurotrophic factor called glial cell-derived neurotrophic factor (or GDNF). But GDNF only represents a small fraction of a much larger class of neurotrophic factors, called the Transforming growth factor-β (TGF-β) superfamily. Recently, researchers have been investigating some of the other TGF-β family members in preclinical models of Parkinson’s and they have been making some interesting discoveries. In today’s post, we will discuss what is meant by neurotrophic factor, explore who else is in the TGF-β superfamily, and look at two recent reports highlighting family members in the context of Parkinson’s. # # # # |
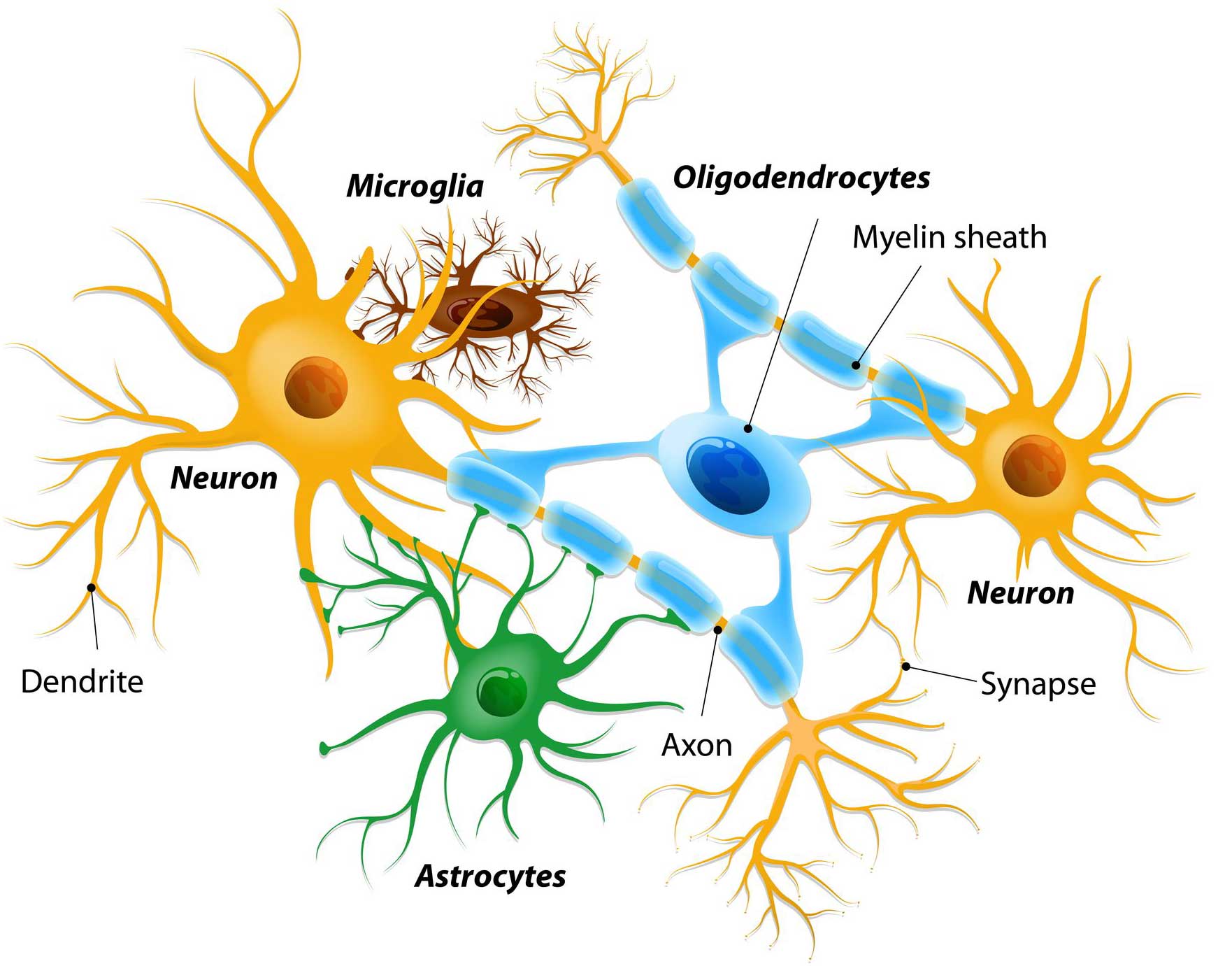
Glial cells are the support cells in the brain. While neurons are considered to be the ‘work horses’ of neurological function – passing messages and storing memories – glial cells are in the background making sure that neurons are protected and well nurtured.
There are different types of glial cells, including astrocytes, oligodendrocytes and microglia. And each type has a specific function, for example microglia are the brain’s resident immune cells checking up on the health of the neurons while oligodendrocytes provide the neurons with a protective covering (called myelin sheath) which also helps to speed up the signalling of neurons.

Astrocytes provide nutrients and neurotrophic factors to neurons and make sure the environment surrounding the neurons is balanced and supportive. Glial cells are absolutely critical to the normal functioning of the brain.
What are neurotrophic factors?
Neurotrophic factors (neurotrophic = Greek: neuron – nerve; trophikós – pertaining to food/to feed) are small proteins that nurture neurons and support growth. There are many types of neurotrophic factors, some having more beneficial effects on certain types of neurons and not other.
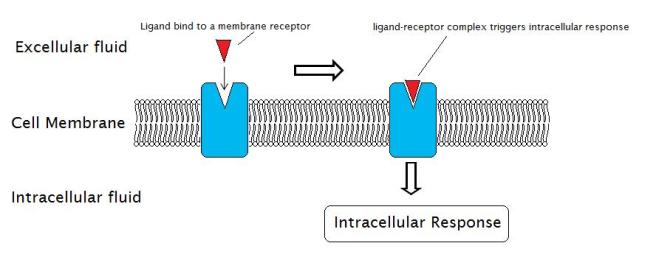
Neurotrophic factors function by being secreted into the extracellular space, where they are able to bind to receptors.
What are receptors?
On the outer surface of cells there are small proteins called receptors, which act like switches for certain biological processes. Receptors will wait for another protein (referred to as a ligand) to come along and activate them. Activation of a receptor results in biological pathways inside the cell being turned on, resulting in an intracellular response.
Can you give me an example of a neurotrophic factor?
Yes. Glial cell-derived neurotrophic factor (or GDNF) is a neurotrophic factors and it has been well studied in the context of Parkinson’s.
We have discussed it at length several times here on the SoPD (Click here and here to read previous SoPD posts dealing with GDNF), but GDNF is a member of a much larger family of neurotrophic factors, called the “Transforming growth factor-β (TGF-β) superfamily“:

The TGF-β superfamily family tree contains approximately 20 secreted messenger proteins that have been evolutionarily conserved across history. As the image above suggests, the family can be subdivided into several sub families, including TGF-βs, bone morphogenetic proteins (BMPs), activins, inhibins, nodals, and growth and differentiation factors (GDFs).
All of them bind to two sets of receptors (Types I and II), which activate a variety of biological pathways inside of cells:

These pathways stimulate growth and survival within a cell, both during development and in situations of stress or damage. As a result of their functions, these neurotrophic factors have been the focus of a lot of research on neuroprotective therapies for neurodegenerative conditions.
For a good review of the TGF-β superfamily in the context of neurological conditions, click here.
|
# RECAP #1: Neurotrophic factors are proteins that nurture and support the development and survival of neurons. They are released by cells and they function by binding to receptors on the outer surface of neurons. This binding activates biological pathways inside cells, which stimulate growth and development. One family of neurotrophic factors is called the Transforming growth factor-β (TGF-β) superfamily. Members of this family have been investigated in models of Parkinson’s. # |
So in the intro you mentioned new research on members of the TGF-β superfamily in models of Parkinson’s?
Yes. Recently two report have been published back-to-back in the same research journal.
This was the first study:

Authors: Goulding SR, Concannon RM, Morales-Prieto N, Villalobos-Manriquez F, Clarke G, Collins LM, Lévesque M, Wyatt SL, Sullivan AM, O’Keeffe GW.
Journal: Brain. 2020 Nov 30:awaa367. Online ahead of print.
PMID: 33253375
In this study, the researchers had 3 goals:
- To investigate whether high levels of Parkinson’s associated alpha synuclein protein could reduce levels of another protein called Ret on dopamine neurons.
- Examine if high levels of alpha synuclein protein could affect the three key signalling mediators of the neurotrophic actions of growth differentiation factor 5 (GDF5, also known as bone morphogenetic protein 14 (or BMP14).
- Examine if a RET-independent neurotrophic factor (such as GDF5) can rescue models of Parkinson’s.
Any questions?
Yeah, what does any of that mean? Like what was Ret???
Ret proto-oncogene (or Ret) is the receptor of GDNF and in 2012, a research report was published which indicated that high levels of the Parkinson’s-associated alpha synuclein protein resulted in the reduction of Ret protein in dopamine neurons.
Here is that report:

Authors: Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Björklund A.
Journal: Sci Transl Med. 2012 Dec 5;4(163):163ra156.
PMID: 23220632 (This report is OPEN ACCESS if you would like to read it)
This finding raised questions as to whether GDNF would work as a therapeutic in the brain of people with Parkinson’s – if high levels of alpha synuclein reduce Ret, will GDNF be able to have any function in the Parkinsonian brain (which classically have a build up of alpha synuclein protein)?
In this 2012 study, the researchers found that GDNF could not protect dopamine neurons in an alpha synuclein model of Parkinson’s (and this result was supported by the findings of an independent research group – click here to read more about that). The researchers found that GDNF was unable to have its effect because the high levels of alpha synuclein were reducing the levels of Ret.
And this new study being disucssed in this post today (Goulding et al 2020) also found that high levels of alpha synuclein in the dopamine neurons of rats resulted in a reduction of Ret protein (but not as much as the original 2012 report).
Ok, I now understand that Ret part, but what about the second goal: the “three key signalling mediators of the neurotrophic actions of growth differentiation factor 5 (GDF5, also known as bone morphogenetic protein 14 (or BMP14)“?
Like GDNF, Growth differentiation factor 5 (or GDF5) is a member of the TGF-β superfamily.

And like GDNF, GDF5 has previously been reported to have neuroprotective properties in neurotoxin models of Parkinson’s (Click here to read about an example of that research), even when delayed administration was applied (Click here to read more about this).
Unlike GDNF, however, GDF5 does not require Ret to have its neuroprotective effect. Rather it acts via a receptor complex, which consists of 3 proteins: bone morphogenetic protein receptor 2 (or BMPR2), bone morphogenetic protein receptor 1B (or BMPR1B, and another protein called SMAD1.
In this new report, the researchers wanted to check if high levels of alpha synuclein reduced the levels of these three proteins.
They reported that it did not. High levels of alpha synuclein had no impact on the levels of BMPR2, BMPR1B, or SMAD1 protein.
Ok, so Parkinson’s associated alpha synuclein protein does not affect the GDF5 pathway?
That is what the data suggests.
And this led the researchers to shift their attention to their third goal: Examine if a RET-independent neurotrophic factor (such as GDF5) can rescue models of Parkinson’s.
To do this, the investigators used a virus that was engineered to make cells produce high levels of alpha synuclein. When they infected the dopamine neurons of rats with this virus, it caused the dopamine neurons to slowly die.
In half of the animals that received the , however, the investigators also injected a carefully designed virus that produced high levels of GDF5.
They found that the animals treated with both alpha synuclein virus and the GDF5-virus exhibited no loss of dopamine neurons. Even when the GDF5 virus was injected in the striatum (infecting cells in the brain region where dopamine neurons project their branches to), the researchers found that this neurotrophic factor could have a neuroprotective effect.
The researchers concluded their report by saying that “The observed neuroprotective effects of GDF5 in this preclinical model support the theory that Ret-independent neurotrophic factors may have therapeutic benefit in Parkinson’s disease and are worthy of further exploration“.
|
# # RECAP #2: Growth differentiation factor 5 (or GDF5) is a neurotrophic factor that has been reported to have beneficial properties in models of Parkinson’s. Recently researchers demonstrated that administration of a gene therapy-based GDF5 in a model of Parkinson’s was able to prevent dopamine neuron cell loss. # # |
At the top of this post you said there were two recent reports. What does the second study say?
The second study was published in the same journal as the report discussed above, but it explores a difference member of the TGF-β superfamily.
Here is the second report:

Authors: Vitic Z, Safory H, Jovanovic VM, Sarusi Y, Stavsky A, Kahn J, Kuzmina A, Toker L, Gitler D, Taube R, Friedel RH, Engelender S, Brodski C.
Journal: Brain. 2020 Nov 30:awaa368. Online ahead of print.
PMID: 33253359 (This report is OPEN ACCESS if you would like to read it)
In this study, rather than investigating GDF5, the researchers evaluated BMP5/7.
What is BMP5/7?
The correct question is “what are BMP5/7?” because it is actually referring to two different proteins.
Opps. Sorry. What are BMP5/7?
BMP5 and BMP7 are two members of the TGF-β superfamily – both are secreted proteins with neurotrophic properties, like GDF5 and GDNF.
The researchers behind this study had previously investigated BMP5 and BMP7 in the development of dopamine neurons in mice. They had found that if they engineered mice that do not produce BMP5 and BMP7 in the brain, those mice did not have any dopamine neurons. Everything else was basically there, but no dopamine neurons.
Here is that report:

Authors: Jovanovic VM, Salti A, Tilleman H, Zega K, Jukic MM, Zou H, Friedel RH, Prakash N, Blaess S, Edenhofer F, Brodski C.
Journal: J Neurosci. 2018 Feb 14;38(7):1662-1676.
PMID: 29321139 (This report is OPEN ACCESS if you would like to read it)
This curious discovery naturally got the researchers wondering if BMP5/7 might be important for the long-term survival of dopamine neurons, and if so, could this combination be used as a neuroprotective strategy for a condition like Parkinson’s? Their idea was supported by a research report from an independent group of scientists who found that administration of BMP7 alone in a model of Parkinson’s was partly neuroprotective (Click here to read more about that study).
To test the idea of a BMP5/7 combo, the researchers generated three viruses. The first virus (like the GDF5 study discussed above) generated high levels of the alpha synuclein protein – ultimately killing the dopamine neurons it infects. The second and third viruses produced high levels of BMP5 and BMP7, respectively.
When the researchers injected the alpha synuclein virus by itself into the brains of mice – into a region called the substantia nigra, where the dopamine neurons reside – the dopamine neurons started dying.
You can see in the image below what the dopamine neurons in the substantia nigra (red dots) should look like in the “control” panel (no virus injected). When the alpha synuclein virus alone was injected, there were less red dots (indicating that dopamine neurons have been lost – in the “a-syn” panel). But when the alpha synuclein virus was injected together with the BMP5 virus and the BMP7 virus (all three viruses together – the “a-syn+BMP5/7” panel), the substantia nigra looks similar to the ‘control’ panel, suggesting that the dopamine neurons are protected and surviving.

The loss of cells dopamine neurons in the substantia nigra results in a loss of dopamine fibres in an area of the brain called the striatum. As I mentioned above, this region is where dopamine neurons project their branches (called axons) to. As you can see in the image below, when the alpha synuclein virus alone was injected, there were less red colouration in the striatum (suggesting dopamine fibres have been lost along with the dopamine neurons – in the “a-syn” panel). But when the alpha synuclein virus was injected together with the BMP5 virus and the BMP7 virus (all three viruses together – the “a-syn+BMP5/7” panel), the colouration in the striatum looks similar to the ‘control’ panel, suggesting that the dopamine fibres are protected and surviving.

In further analysis, the investigators found that the BMP5/7 virus treatment not only protected the dopamine neurons, but it also reduced the immune response (lower levels of activated microglia and astrocytes) and it rescued Parkinson’s-like motor problems in the mice.
Interestingly, the researchers finished their study by looking at levels of Smad6 protein in the brains of people with Parkinson’s.
What is Smad6?
SMAD Family Member 6 (or Smad6) is a protein that belongs to a family of TGFβ superfamily modulators. It is a protein that inhibits BMP5 and BMP7 activity.
Smad6 was recently found to be elevated in the postmortem substantia nigra of people with Parkinson’s (Click here to read more about this). And in this current study, the researchers also found that Smad6 levels were elevated in the postmortem substantia nigra of people with Parkinson’s. This last finding suggests that BMP signalling may be partly disrupted in some individuals with PD – this will require further analysis.
The researchers concluded their report by saying “that neurotrophic factors remain a valid approach and that dose adjustments, progress in drug delivery and testing new drug candidates, including BMPs, are promising strategies to establish a disease-modifying therapy for Parkinson’s“.
So what does it all mean?
Here on the SoPD, I regularly talk about any ‘curative therapy’ for Parkinson’s requiring three core components:
- A disease halting mechanism (to slow down or stop the progression of the condition)
- A neuroprotective agent (to protect and rejuvenate the remaining cells)
- Some form of restorative therapy (to replace the lost cells and function)
Neurotrophic factors have long been looked upon as part of that second component (protecting the remaining stressed cells and helping to restore them back to health), possibly even overlapping with the third component (encouraging some regrowth in the remaining cells).
Much of the previous neurotrophic factor research in Parkinson’s, however, has centred around a small number of proteins (namely GDNF and Neurturin – click here and here to read previous SoPD posts about these two). Thus, it is extremely encouraging to see the research highlighted in this post investigating completely different neuroprotective factors.
Although this research is still a long way from clinical testing, it is good to see fresh options being proposed and explored. More research will be required before attempting to translate any of them to the clinic, but hopefully – with the previous experiences and lessons learned with GDNF and Neurturin – that road will be quicker and more smooth.
We look forward to seeing what happens next with this research.

Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Twitter.


