Improving clinical trials for Parkinson’s

Taking part in research is vital for science to progress, for the people affected by a health condition, and for future generations.
Health research and clinical trials is more than taking medications and spending time being assessed; it involves many stakeholders including patients, carers, clinicians, funding organisations and governing bodies in prioritising, designing and delivering research results.
At Cure Parkinson’s we are focused on improving the clinical trials process to expedite better treatments for people living with Parkinson’s.
More information about improving the clinical trials process
The study of diabetes data to inform future Parkinson’s clinical trials Multi-Arm Multi-Stage – a new approach to clinical trials Research focus: The International Linked Clinical Trials programme The International Linked Clinical Trials (iLCT) programme
Moving towards a new staging system for Parkinson’s
Cure Parkinson’s has been a part of a group of Parkinson’s scientists, charity organizations, and patient advocates from around the world to help develop a new biological staging framework…

Exploring Perspectives on Trial Design for Parkinson’s: An Update on the Delphi Study
Recently, Cure Parkinson’s was pleased to see the results of one of our funded studies published in the Journal of Parkinson’s Disease.

Our research impact
Our recent research impact report determines and highlights the impact our research funding has had on the Parkinson’s research field.

The new Multi-arm Multi-stage MAMS trial model for Parkinson’s
One of the most frustrating aspects of the way we currently conduct clinical trials is the long delays between studies. Multi-Arm, Multi-Stage (or MAMS) clinical trials may represent a…

Research Update Meeting Spring 2023 – recorded to watch again
Our popular and much-awaited research update meeting brought together two leading clinicians to report on their individual area of expertise for Parkinson’s. It’s available to watch again!

The 2022 International Linked Clinical Trials meeting
Cure Parkinson’s annually coordinates the International Linked Clinical Trials (iLCT) meeting to rank and prioritise therapies that may have the potential to modify the course of Parkinson’s progression.
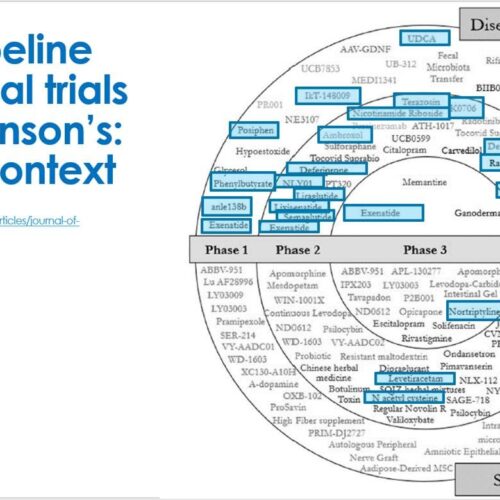
The 2022 Parkinson’s drug development pipeline report
The 2022 annual report, outlining the current development pipeline of new drug-based treatments for Parkinson’s, has been published.

Parkinson’s Clinical Study Group
Cure Parkinson’s is funding Professor Oliver Bandmann at the University of Sheffield to establish a nationwide infrastructure for a study group focused specifically on conducting clinical trials for Parkinson’s….

Improving Parkinson’s Trials: A Delphi Study
Under the visionary leadership of Dr Camille Carroll at the University of Plymouth, Cure Parkinson’s has been supporting an exploratory study at the University of Plymouth to understand the…

Multi-Arm Multi-Stage – a new approach to clinical trials
One of the most frustrating aspects of clinical trials is the long delays between studies. Dr Camille Carroll is leading a group of researchers exploring the use of multi-arm…

Multi-Arm Multi-Stage trials for Parkinson’s
At present, most clinical trials for Parkinson’s involve a single therapy, being tested against a ‘placebo’ or control treatment for a fixed period of time. At the end of…
