New iLCT Pipeline study: Theracurmin

Cure Parkinson’s is excited to announce the funding of the fourth preclinical project as part of our iLCT Pipeline Research Acceleration Programme.
Led by Dr Ayse Ulusoy at the German Center for Neurodegenerative Diseases (DNZE), this preclinical project will evaluate Theracurmin in models of Parkinson’s to determine whether it is able to slow progression.
What is Theracurmin?
Theracurmin is a modified form of curcumin, a compound naturally found in turmeric. Many studies have shown the beneficial effects of curcumin, particularly its anti-inflammatory and antioxidant properties. Both inflammation and oxidative stress – cell stress caused by the build-up of toxic molecules – are drivers of Parkinson’s progression. Therefore, if a drug can address these, it could be a suitable treatment for Parkinson’s. Curcumin has also been investigated for other conditions, such as cancer, inflammatory bowel disease (IBD), and Type 2 diabetes.
Why are we interested in Theracurmin and not curcumin?
When we take a pill or tablet, it is broken down by our digestive system to allow the active ingredients in the medication to be absorbed into the bloodstream and distributed throughout the body. The amount of a drug that reaches circulation is referred to as its “bioavailability”.
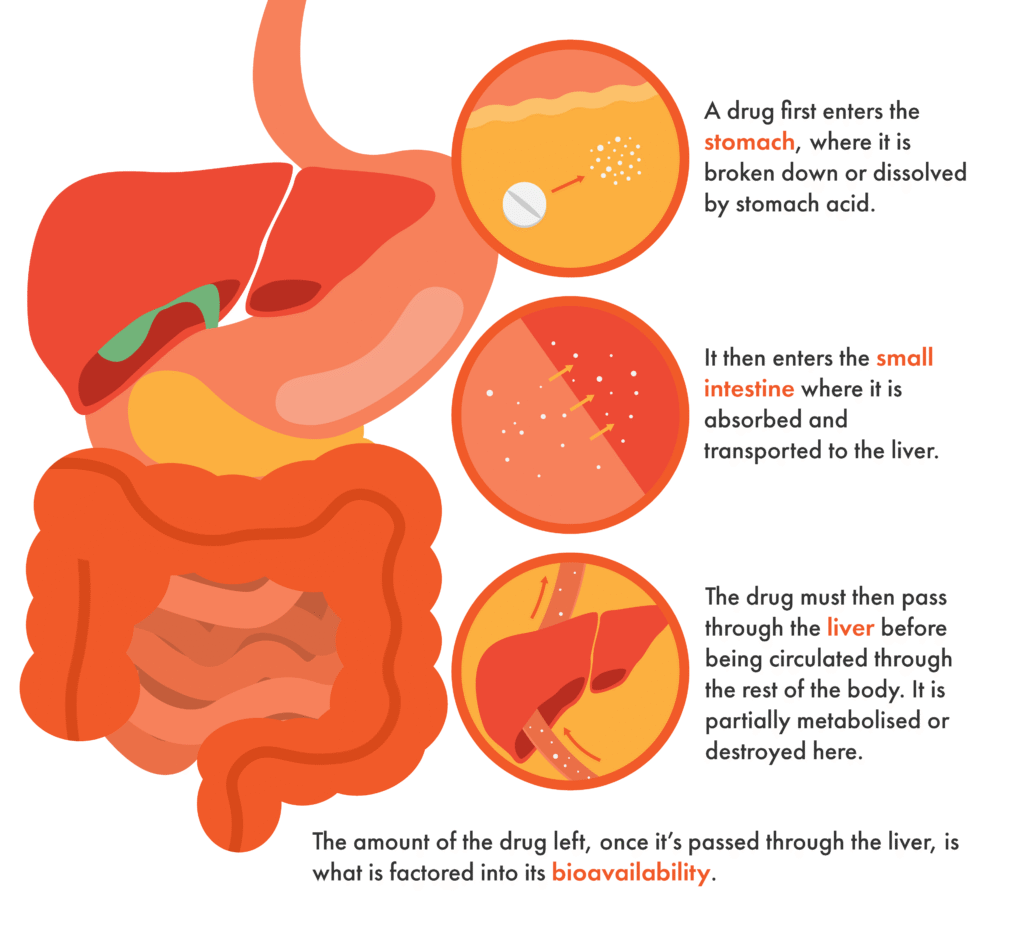
This process can present some challenges, however. Curcumin, for example, has low bioavailability since it is broken down quickly by our digestive system and not easily absorbed by our intestines. This means that on its own, curcumin would not be a suitable treatment for Parkinson’s, as very little of it reaches the bloodstream and ultimately gets to the brain.
Theracurmin is a slow-release formulation of curcumin that is thought to be more bioavailable. Early-stage clinical trials involving healthy volunteers have provided evidence towards this, indicating that Theracurmin is much more readily absorbed when compared to normal curcumin powder [1].
How do we make drugs more bioavailable?
To overcome the issue of bioavailability, drugs will often contain additional inactive ingredients. These can help slow or improve breakdown and increase absorption, or they can serve other functions like improving taste.
Researchers may also change the physical or chemical structure of a medication. In the case of Theracurmin, curcumin has been modified to be a much smaller molecule, making it easier for the body to absorb.
Why is this study important?
Although we know a lot about curcumin, Theracurmin has yet to be studied in models of Parkinson’s. Before we can progress Theracurmin into a clinical trial, we need to know whether it is safe and effective. It is because of this that the International Linked Clinical Trials (iLCT) committee recommended the need for additional research when Theracurmin was evaluated in 2019.
There are two phases to this study; the first will be to assess the bioavailability of Theracurmin, specifically how much of it reaches the bloodstream and brain. The second part will be looking at whether Theracurmin is protective against several drivers of Parkinson’s progression, including the accumulation of the protein alpha-synuclein, inflammation, and oxidative stress. We are hopeful that the results of this study will inform us how best to proceed with the compound as a potential treatment for Parkinson’s.