The Hope List by Dr Kevin McFarthing
The Hope List collated by Dr Kevin McFarthing details information about current potential therapies in research and clinical stages. Originally a PhD biochemist, Kevin now chairs the Patient and Public Involvement and Engagement group for the Edmond J Safra Accelerating Clinical Trials in Parkinson’s Disease programme; he sits on the Cure Parkinson’s Research Committee and is joint author of the annual pipeline paper. Kevin was diagnosed with Parkinson’s in 2012 at the age of 55.
*Note: The spreadsheet is via Google Drive and not always compatible with mobile devices. We would suggest viewing the spreadsheet on a laptop or similar.
Key highlights from the report
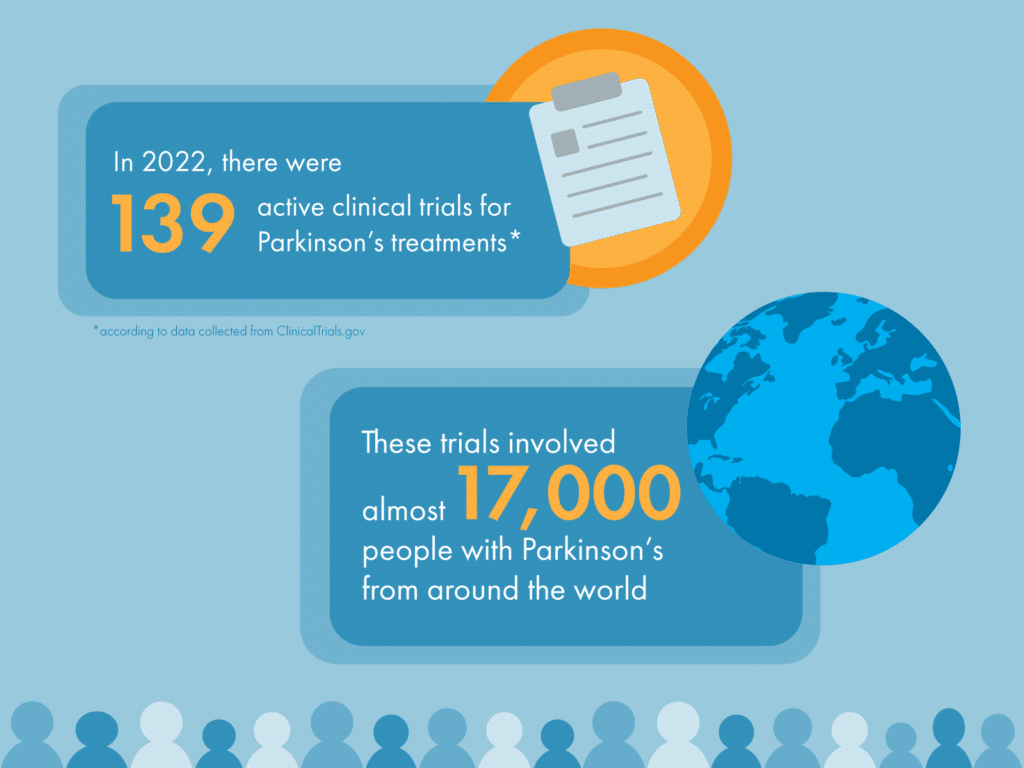
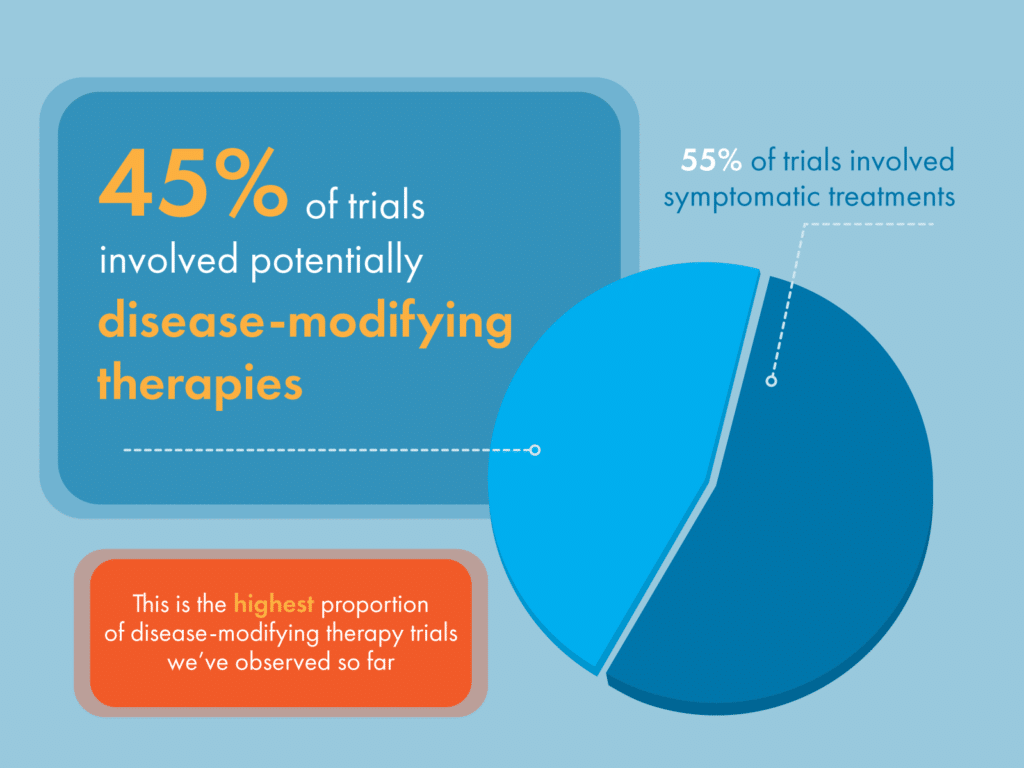
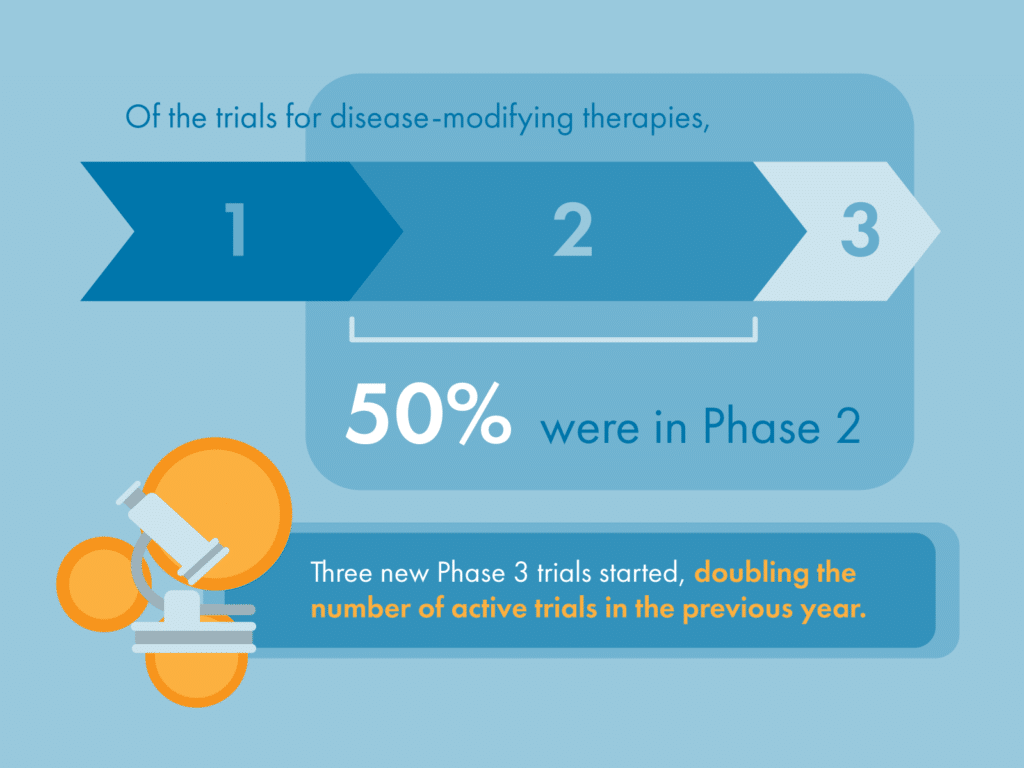
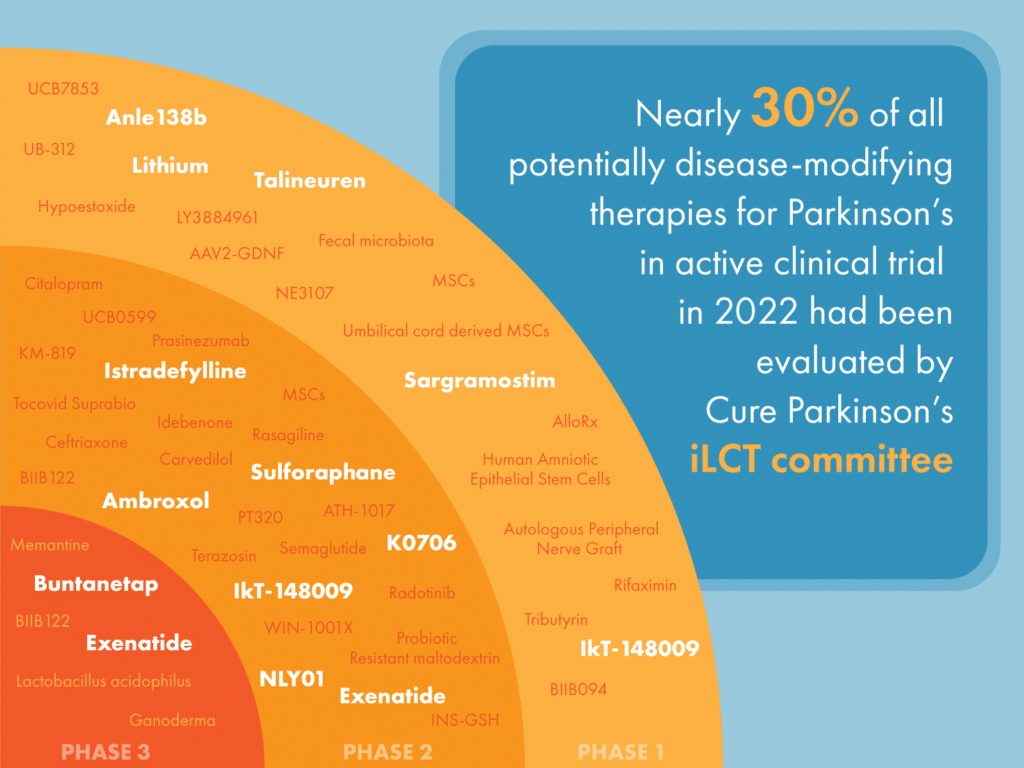
We’re delighted to see nearly 30% (15/52) of all potentially disease-modifying therapies for Parkinson’s in active clinical trial up until January 31 2023 have been evaluated by Cure Parkinson’s iLCT committee
Dr Simon Stott, Director of Research, Cure Parkinson’s