|
# # # # Glucagon-like peptide 1 receptor (or GLP-1R) agonists are a frontline treatment for diabetes – improving glycaemic control by reducing glucose concentrations in the blood. In 2008, multiple research groups reported that this class of drugs exhibited neuroprotective properties in models of Parkinson’s. Subsequent clinical trials have provided encouraging data supporting this assertion. Recently, researchers have found further support for potential beneficial effects in a large epidemiological study. In today’s post, we will discuss what GLP-1R agonists are, what has previously been done with them in Parkinson’s, and what the new report found. # # # # |
In 2012, the Golden Goose Award was awarded to Dr John Eng, an endocrinologist from the Bronx VA Hospital.
Dr John Eng. Source: Health.USnews
The Award was originally created in 2012 to celebrate researchers whose seemingly odd or obscure federally funded research turned out to have a significant and positive impact on society.
And despite the name, it is a very serious award – past Nobel prize winners (such as Roger Tsien, David H. Hubel, and Torsten N. Wiesel) are among the awardees.
Sounds interesting. What did Dr Eng do?
In the 1980’s, Dr Eng became really interested in some studies (such as this one) that described the effects that certain types of venom had on cells in the pancreas.
Remind me, what is the pancreas?
The pancreas is the organ that produces the chemical insulin, which is critical for maintaining normal glucose levels in our bodies. The pancreas is located below the stomach.

Previous research suggested that venom could stimulate the pancreas to release insulin. Having worked with diabetic people who do not produce enough insulin, Dr Eng started wondering if venom may useful for people with diabetes. But rather than simply injecting diabetic people with venom, he started looking at all the chemicals that make up the venom from different poisonous creatures.
What did he find?
This charming creature is a Gila monster.
The Gila monster. Source: Californiaherps
Cute huh?
Some interesting facts about the Gila (pronounced ‘Hila’) monster:
- They are named after the Gila River Basin of New Mexico and Arizona (where these lizards were first found)
- They are protected by State law
- They are venomous, but very sluggish creatures
- They spend 95 percent of their time underground in burrows.
In 1992, Dr Eng identified the two proteins that he had isolated from the venom of the Gila monster. One of them was called exendin-4 and it bore a striking similarity – structurally and functionally – to a human protein, called glucagon like peptide-1 (or GLP-1).
What is GLP-1?
Insulin instructs cells to take in and use glucose from the blood. This has the effect of lowering blood sugar. The hormone Glucagon has the opposite effect – it tells the body to release glucose into the blood to raise sugar levels.
GLP-1 is a hormone that stimulates insulin production while blocking glucagon release.
The function of GLP-1. Source: Wikipedia
Unfortunately, naturally produced GLP-1 in your body is rapidly deactivated by a circulating enzyme called dipeptidyl peptidase IV (or DPP-4, remember this name – we’ll come back to it later). Exendin-4, however, was found to be resistant to this deactivation, meaning that could last longer in the body stimulating insulin production and blocking glucagon release.
Dr Eng quickly realised that there was enormous medicinal potential for exendin-4 as a drug for people with diabetes. He patented the idea and soon afterwards a biotech company called Amylin Pharmaceuticals to begin the work of turning exendin-4 into a drug for diabetes.

That drug was eventually called Exenatide.

In April 2005, Byetta (the commercial name for Exenatide) was approved by the FDA for clinical use in the treatment of Type 2 diabetes. On the 27th January 2012, the FDA gave approval for a new formulation of Exenatide called Bydureon, as the first weekly treatment for Type 2 diabetes. In July of 2012, Bristol-Myers Squibb announced it would acquire Amylin Pharmaceuticals for $5.3 billion, and one year later AstraZeneca purchased the Bristol-Myers Squibb share of the diabetes joint venture.
|
# RECAP #1: Glucagon like peptide-1 (or GLP-1) is a hormone in the body that stimulates insulin production. Synthetic versions of it can be used to treat individuals with Type 2 diabetes. Biotech companies have developed drugs that act like GLP-1 – the most well known of these is called exenatide. # |
Interesting, but what does any of this have to do with Parkinson’s?
In 2008, this report was published:
Title: Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease.
Authors: Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H, Wikström L.
Journal: J Neurosci Res. 2008 Feb 1;86(2):326-38.
PMID: 17803225
In this study, Exendin-4 (the protein very similar to exenatide) was tested in a rat model of Parkinson’s. Five weeks after giving the neurotoxin (6-hydroxydopamine) to the rats, the investigators began treating the animals with exendin-4 over a 3 week period. Despite the delay in starting the treatment, the researchers found behavioural improvements and a reduction in the number of dying dopamine neurons.
And this first result was followed a couple of months later by a similar report with a very similar set of results:
Title: Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease.
Authors: Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS.
Journal: J Neuroinflammation. 2008 May 21;5:19. doi: 10.1186/1742-2094-5-19.
PMID: 18492290 (This study is OPEN ACCESS if you would like to read it)
The scientists in this study tested exendin-4 on two different rodent models of Parkinson’s (6-hydroxydopamine and lipopolysaccaride), and they found similar results to the previous study. The drug was given 1 week after the animals developed the motor features, but the investigators still reported positive effects on both motor performance and the survival of dopamine neurons.
And the following year, in 2009, two more research reports were published suggesting that exendin-4 was having positive effects in models of Parkinson’s (Click here and here to read those reports).
This was a lot of positive results for this little protein.
How is Exendin-4/Exenatide having this positive effect?
Exendin-4 and exenatide are both GLP-1 receptor agonists.
What does that mean?
On the surface of cells there are small proteins called receptors, which act like switches for certain biological processes. Receptors will wait for another protein to come along and activate them or alternatively block them.
The proteins that activate the receptors are called agonists, while the blockers are called antagonists.
Agonist vs antagonist. Source: Psychonautwiki
Exendin-4 and exenatide are agonists, so they activate the GLP-1 receptor.
Activation of the GLP-1 receptor by a GLP-1 receptor agonist like exendin-4 or exenatide results in the activation of many different biological pathways within a cell:
The GLP-1 signalling pathway. Source: Sciencedirect
Of particular importance is that GLP-1 receptor activation inhibits cell death pathways, reduces inflammation, reduces oxidative stress, and increases neurotransmitter release. All pretty positive stuff really (Click here for a very good OPEN ACCESS review of the GLP-1-related Parkinson’s research field).
And all of these preclinical research reports with positive results led to and supported the idea of clinically testing exenatide in people with Parkinson’s.
What happened in the first clinical trial?
The first clinical trial of exenatide in Parkinson’s was a phase I trial to determine if the drug was safe to use in people with Parkinson’s. The results of the trial were published in 2013:
Title: Exenatide and the treatment of patients with Parkinson’s disease.
Authors: Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T.
Journal: J Clin Invest. 2013 Jun;123(6):2730-6.
PMID: 23728174 (This study is OPEN ACCESS if you would like to read it)
The researchers gave exenatide (the Byetta formulation which is injected twice per day) to a group of 21 people with moderate Parkinson’s and evaluated their progress over a 14 month period. They compared those participants to 24 additional subjects with Parkinson’s who acted as control (they received no treatment). Exenatide was well tolerated by the participants, although there was some weight loss reported among many of the subjects (one subject could not complete the study due to weight loss).
Importantly, the exenatide-treated subjects demonstrated improvements in their Parkinson’s movement symptoms (as measured by the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (or MDS-UPDRS)), while the control patients continued to decline.
Interestingly, in a two year follow up study – which was conducted 12 months after the subjects stopped receiving exenatide – the researchers found that participants previously exposed to exenatide demonstrated a significant improvement (based on a blind assessment) in their motor features when compared to the control subjects involved in the study.
It is important to remember, however, that this trial was an ‘open-label study’ – that is to say, the participants knew that they were receiving the exenatide treatment so there is the possibility of a placebo effect explaining the improvements. And this necessitated the testing of the efficacy of exenatide in a phase II double blind clinical trial.
And the results of that trial were published last August (2017):

Authors: Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T
Journal: Lancet 2017 Aug 3. pii: S0140-6736(17)31585-4.
PMID: 28781108 (This report is OPEN ACCESS if you would like to read it)
In the study, the investigators recruited 62 people with Parkinson’s (average time since diagnosis was approximately 6 years) and they randomly assigned them to one of two groups, exenatide (the Bydureon formulation which is injected once per week) or placebo (32 and 30 people, respectively). The treatment was given for 48 weeks (in addition to their usual medication) and then the participants were followed for another 12-weeks without exenatide (or placebo) in a ‘washout period’.
It is important to remember that in this trial everyone was blind. Both the investigators and the participants. This is referred to as a double-blind clinical trial and is considered the gold standard for testing the efficacy of a new drug.
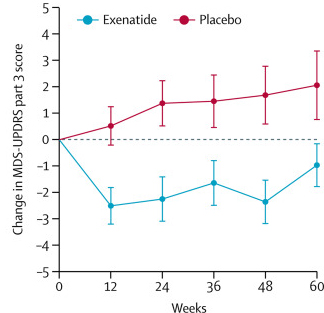
The researchers found a statistically significant difference in the motor scores of the exenatide-treated group verses the placebo group (p=0·0318). As the placebo group continued to have an increasing (worsening) motor score over time, the exenatide-treated group demonstrated improvements, which remarkably remained after the treatment had been stopped for 3 months (weeks 48-60 on the graph below).
Reduction in motor scores in Exenatide group. Source: Lancet
Brain imaging (DaTScan) also suggested a trend towards reduced rate of decline in the exenatide-treated group when compared with the placebo group. Interestingly, the researchers found no significant differences between the exenatide and placebo groups in scores of cognitive ability or depression – suggesting that the positive effect of exenatide may be specific to the dopamine or motor regions of the brain.
Given that these results were coming from a randomised, double-blind clinical trial, the Parkinson’s community got rather excited about them (Click here to read a previous SoPD post about this particular trial).
A Phase III clinical trial of exenatide (again, the Bydureon formulation) is now underway at 6 sites in the UK (Click here to read more about this).
Interesting. Are there other clinical trials of GLP-1 agonists in Parkinson’s?
By my count there are at least eight ongoing clinical trials of GLP-1 agonists for Parkinson’s (and I am happy to be corrected on this). They are the:
- Phase III study of Exenatide in Parkinson’s study in the UK
- Phase II study of Liraglutide in Parkinson’s study in Los Angeles
- Phase II study of Lixisenatide in Parkinson’s study in France
- Phase II study of Semaglutide in Parkinson’s study in Norway
- Phase II study of NLY01 in Parkinson’s study across the USA (being conducted by Neuraly)
- Phase II study of PT320 in Parkinson’s in South Korea (being conducted by Peptron)
- Phase II study of Exenatide trial in Parkinson’s in Sweden
- Phase I study of Exenatide in Parkinson’s study in Florida
In addition, I am aware of other interested parties looking to enter this space, so over the next few years we will be seeing a great deal of clinical trial data dealing with GLP-1R agonists in Parkinson’s.
And if the clinical data suggests that this class of drug is having an impact on Parkinson’s, we may also see the appearance of dual agonists.
Dual agonists?!?
Dual agonists are agents that are capable of activating two receptors. Diabetes researchers have been developing dual agonists of both GLP-1R and the hormone glucose-dependent insulinotropic polypeptide (or GIP) receptor.
Like GLP-1, GIP also stimulates insulin secretion (Click here to read more about this). And like GLP-1, agonists of GIP have been reported to be neuroprotective in models of Parkinson’s (Click here and here to read more about this).
Given that GLP-1 and GIP receptor agonists are both having positive effects in PD models, researchers have recently developed a dual agonist that can cross the blood brain barrier (the protective membrane surrounding our brains).
And very recently, the results of a preclinical study were published and they indicated that the dual agonist approach may be more potent that GLP-1R agonism alone in a model of PD.
This is the report here:

Authors: Zhang L, Zhang L, Li Y, Li L, Melchiorsen JU, Rosenkilde M, Hölscher C.
Journal: J Parkinsons Dis. 2020;10(2):523-542.
PMID: 31958096
In this study, the researchers used a classical neurotoxin model (MPTP) of Parkinson’s and compared a new GLP-1/GIP receptor dual agonist (called DA-CH5) with a well characterised GLP-1R agonist called Liraglutide. They found that both agents improved the motor impairments associated with the model and reversed the loss of dopamine neurons.
In addition, the investigators reported that DA-CH5 was superior to Liraglutide in reducing the activation of microglia and astrocyte (helper cells that maintain the health of neurons), improving energy production in cells (mitochondrial activity), and boosting cellular waste disposal (autophagy).
Dual agonists are now being clinically tested in Type 2 diabetes (Eli Lilly’s Tirzepatide (aka LY3298176) is an example of this – click here and here to read more about this). In addition, a biotech company called Kariya Pharmaceuticals is developing a dual agonist called KP-405, which is being targetted towards neurodegenerative conditions.
|
# # RECAP #2: Parkinson’s researchers have explored the use of exenatide in models of Parkinson’s and found it to have beneficial effects. Initial clinical trials of exenatide in people with Parkinson’s suggest encouraging results on motor symptoms. Next generation agents – which combine the properties of GLP-1 and similar proteins – have been developed for diabetes, and early preclinical research suggests that these could be useful for Parkinson’s as well. # # |
Excellent. Has anyone ever looked to see if exenatide reduces the risk of developing Parkinson’s?
So this is a really interesting question, one that the researchers who conducted the first two clinical trials of exenatide in Parkinson’s wanted to investigate.
And this week they published a report outlining their findings:

Authors: Brauer R, Wei L, Ma T, Athauda D, Girges C, Vijiaratnam N, Auld G, Whittlesea C, Wong I, Foltynie T.
Journal: Brain. 2020 Oct 4:awaa262. Online ahead of print.
PMID: 33011770
In this study, the researchers used data from The Health Improvement Network – a large database of anonymised electronic medical records collected from 808 UK primary care clinics. Basically, it is a repository of anonymised medical information about 15 million people.
Within the data, the investigators identified 100 288 individuals diagnosed with Type 2 diabetes who had been prescribed standard diabetes therapies (glitazones, GLP-1R agonists, and/or dipeptidyl peptidase 4 (DPP-4) inhibitors) on or after the 1st January 2006.
What are glitazones?
Glitazones (also known as thiazolidinediones) is another class of licensed diabetes drug. It reduces insulin resistance by increasing the sensitivity of cells to insulin.
Glitazone has been shown to offer protection in animal models of Parkinson’s (Click here and here for more on this). And this class of drug has been clinically tested in Parkinson’s (Click here to read the results of that study).
And dipeptidyl…whatever DPP-4 inhibitors?
Again, another type of diabetes treatment (Click here to read an old SoPD post on DPP-4). DPP-4 is a rather indiscriminate enzyme, breaking down a wide range of proteins. A particular protein will do its job and then DPP-4 will come in chew it up and get rid of it. The proteins targeted by DPP-4 include:
- Glucagon
- Glucagon-like peptide-1 (GLP-1)
- Glucagon-like peptide-2 (GLP-2)
- Glucose-dependent insulinotropic polypeptide (GIP)
Thus, by inhibiting DPP-4, scientists have been able to raise natural levels of proteins that are useful in diabetes, providing a powerful treatment for managing the condition. The diabetes treatment alogliptin represents a DPP-4 inhibitor.
Ok, so the researchers identified lots of people who took glitazones, GLP-1R agonists, and/or DPP-4 inhibitors. Then what did they do?
Of the 100, 288 identified cases, there were:
- 21 175 individuals who were prescribed glitazones;
- 36 897 individuals who were prescribed DPP4 inhibitors;
- 10 684 individuals who were prescribed DPP4 inhibitors GLP-1 agonists (6861 of whom were prescribed GTZ and/or DPP4 prior to using GLP-1 mimetics)
Among 100, 288 cases, there were 329 (0.3%) people diagnosed with Parkinson’s.
When the investigators looked at these particular individuals, they found that the use of DPP-4 inhibitors and/or GLP-1R agonists was associated with a lower rate of Parkinson’s (compared to the use of other diabetic treatments).
Specifically, the incidence rate ratio was 0.64 for DPP-4 inhibitors (95% confidence interval 0.43-0.88; P < 0.01) and 0.38 for GLP-1R agonists (95% confidence interval 0.17-0.60; P < 0.01).
The researchers concluded that the incidence of Parkinson’s in patients diagnosed
with Type 2 diabetes “varies substantially depending on the treatment for diabetes received“.
Prof Foltynie who led the study (and is the lead investigator on the Phase III clinical trial of exenatide) added “We have added to evidence that exenatide may help to prevent or treat Parkinson’s disease, hopefully by affecting the course of the disease and not merely reducing symptoms, but we need to progress with our clinical trial before making any recommendations” (Source).
Very interesting. Have other reported similar beneficial effects for GLP-1R agonists?
Not in Parkinson’s, but two years ago another large study suggested that GLP-1R agonist use was associated with lower mortality than other diabetes treatments.
Here is the published report:

Authors: Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K.
Journal: JAMA. 2018 Apr 17;319(15):1580-1591.
PMID: 29677303 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators conducted a meta-analysis of 236 clinical trials involving 176 310 diabetic participants. The results suggested that GLP-1 agonists use was associated with lower mortality (both “all-cause” and “cardiovascular mortality”) compared with DPP-4 inhibitors or placebo or no treatment.
It may be worthy of further investigation this GLP-1R agonist stuff.
So what does it all mean?
GLP-1R agonists represent a mainstay of diabetes therapy, but over the last decade there has been increasing evidence that this class of drug may also be of use in Parkinson’s. Initial preclinical results has been supported by encouraging data from intial clinical trials, which has given rise to further clinical evaluations. And more recently, further support has been provided by an analysis of a large medical database which suggests that GLP-1R agonist use may even reduce the risk of developing Parkinson’s.
Exactly how GLP-1R agonists may be having their neuroprotective effect in the models of Parkinson’s is still being determined. The biotech firm Neuraly has previusly published research suggesting that this class of drugs may be inferring neuroprotection primarily via microglia and astrocytes in the brain, rather than through neurons (Click here to read a previous SoPD post on this topic). As ongoing clinical trials evaluate GLP-1R agoinists in individuals with Parkinson’s, there is a great deal of preclinical research being conducted in parallel that is attempting to better nail down the mechanisms of action of these drugs.
If GLP-1R agonists are eventually found to have a disease modifying effect on Parkinson’s, perhaps the Golden Goose Award will not be the only award given to Dr Eng.
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE – The author of this post is an employee of the Cure Parkinson’s Trust who funded the new research report and has supported the clinical development of exenatide for Parkinson’s – so he might be a little bit biased in his views on the topic. The trust has not requested the production of this post, but the author considered it interesting and important to share with the Parkinson’s community.
In addition, the information provided by the SoPD website is for educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from