|
# # # # Alpha synuclein has long been viewed at “Public enemy #1” by the Parkinson’s research community. This sticky, abundant protein starts to cluster (or aggregate) in Parkinson’s. There have been several attempts to reduce levels of the protein floating around outside of cells (using “immunotherapy” approaches) But now clinical research is ramping up to determine if reducing aggregated alpha synuclein levels in the brain could help to slow/stop the progression of the condition. In today’s post, we will look at three different lines of clinical research focused on small molecule inhibitors of alpha synuclein aggregation. # # # # |
When someone mentions the pharmaceutical firm Novartis, it feels like the company has been around forever, but it is actually not that old.
It was created in March 1996 via the merger of two Swiss companies: Ciba-Geigy and Sandoz. The roots of those companies can be traced back more than 250 years, but the combined entity is still a spring chicken compared to many of its major competitors.
The name Novartis results from the combination of two words “Nova Artes”, which means new art and innovation in simple forms, but there is little in what the company does that is ‘simple’. A good example of this was their block buster cancer drug Gleevec/Glivec (imatinib) which was developed by careful “rational drug design” for very specific types of cancer.

The reputation for Swiss precision seems to flow through this company and they are always making very carefully placed bets.
Which makes their news this week rather interesting.
What news did they have?
The company announced that they were forming a global co-development and co-commercialization agreement with the pharmaceutical company UCB for the their Parkinson’s targeted agent UCB0599 (Click here to read the press release).
UCB0599 is a small molecule, alpha synuclein misfolding inhibitor currently in Phase II clinical development.
What is alpha synuclein?
Alpha synuclein sounds like a distant galaxy, but it is one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron. When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure.
When it is first produced, alpha synuclein protein will look something like this:
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). It is believed that alpha synuclein has certain functions as a monomer, but may also have specific tasks as an oligomer.
In Parkinson’s, alpha synuclein appears to mis-fold and clump (or aggregate) together to form what are called amyloid fibrils.
Images of monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein that aggregate together, and then go on to form what we call Lewy bodies.

A Lewy body is referred to as a cellular inclusion, as they are almost always found inside the cell body. They are a characterisitic feature of the Parkinsonian brain.

Given that the aggregation of alpha synuclein is believed to be an important player in the generation of Lewy bodies and the progression of Parkinson’s, researchers have been trying to develop different means of blocking the aggregation.
UCB0599 is an alpha synuclein aggregation inhibitor.
|
# RECAP #1: Alpha synuclein is a protein that clumps together in Parkinson’s and this ‘aggregation’ behaviour is believed to be important in the progression of the condition. Biotech companies – like the pharmaceutical company UCB – have been developing drugs that block the aggregation of this protein. # |
Got it. So what is the deal between UCB and Novartis all about?
Well, the two companies have agreed to co-development and co-commercialization UCB0599, with an upfront payment of US$150 million going to UCB. And if all of the future milestones are reached further potential payments could be as much as US$1.5 billion. If the drug passes clinical testing and receives approval from the various health regulators, UCB will be the commercial lead in Europe and Japan, while Novartis will look after the US and all other territories.
What do we know about UCB0599?
UCB0599 was originally discovered by a biotech company called Neuropore Therapies.
The company was looking for molecules that reduce alpha synuclein toxicity by displacing alpha synuclein from the membrane.
What does that mean?
Alpha synuclein is known to bind to the inside wall of cells (or their membrane). As we discussed above, floating around by itself, the shape of alpha synuclein protein is intrinsically disordered (see panel A below), but once it interacts with the membrane it adopts a specific structure (called an alpha helical structure – see panel B below). This process allows the protein to facilitate activity involved with cell-to-cell communication (synaptic vesicle fusion and release).

At high concentrations, however, membrane-bound alpha synuclein can disrupt lipid membranes, which has been suggested as a possible mechanism of toxicity. In addition, membrane-bound alpha synuclein can adopt a structure that encourages oligomers to form, which may lead to the aggregated form of alpha synuclein we discussed above.
The researchers at Neuropore Therapies used molecular modelling techniques to design a molecule for the task of displacing alpha synuclein from the membrane.
They called this molecule NPT100-18A.
In 2016, they published this report describing the development and characterisation of NPT100-18A:

Authors: Wrasidlo W, Tsigelny IF, Price DL, Dutta G, Rockenstein E, Schwarz TC, Ledolter K, Bonhaus D, Paulino A, Eleuteri S, Skjevik ÅA, Kouznetsova VL, Spencer B, Desplats P, Gonzalez-Ruelas T, Trejo-Morales M, Overk CR, Winter S, Zhu C, Chesselet MF, Meier D, Moessler H, Konrat R, Masliah E.
Journal: Brain. 2016 Dec;139(Pt 12):3217-3236.
PMID: 27679481 (This report is OPEN ACCESS if you would like to read it)
The researchers found that in the presence of NPT100-18A (red pentagon in the schematic below) the conformation of alpha synuclein changed, resulting in its release from the membrane (the grey collection of circle in the schematic below).
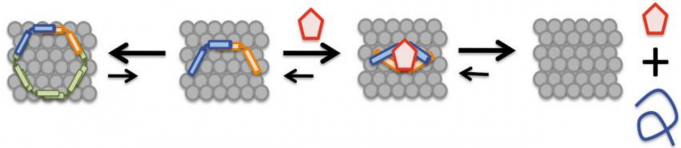
And they found that NPT100-18A improved motor problems, decreased the accumulation of alpha synuclein, and limited the neurodegeneration that is seen in the brains of multiple genetic mouse model of Parkinson’s (the mThy1-α-syn WT and mThy1-α-syn E57K models).
This proof-of-principle result was very encouraging for the Neuropore Therapies investigators who began developing a next generation version of the molecule.
Why? Why didn’t they just take NPT100-18A into clinical trials?
NPT100-18A had limited oral bioavailability (meaning it didn’t get into and last long in the body) and relatively poor brain penetration (<11% of plasma levels), so it would have been difficult to take into clinical trial. The next generation molecule that Neuropore Therapies developed, however, was much better.
It was called NPT200-11.
The development and characterisation of NPT200-11 is described in this publication:
Authors: Price DL, Koike MA, Khan A, Wrasidlo W, Rockenstein E, Masliah E, Bonhaus D.
Journal: Sci Rep. 2018 Nov 1;8(1):16165.
PMID: 30385782 (This report is OPEN ACCESS if you would like to read it)
The results of this study demonstrated that NPT200-11 was a safe molecule with more properties suitable for clinical evaluation (than NPT100-18A). NPT200-11 also reduced alpha synuclein pathology in a genetic mouse model of Parkinson’s (the Line 61 mouse model overexpressing human wild type alpha synuclein).
Further analysis also demonstrated that NPT200-11 reduced neuroinflammation, normalized levels of the dopamine-related proteins (such as DAT) in the brain, and improved motor function in the mice.
So what happened next?
In January 2015, Neuropore Therapies granted a license to the pharmaceutical company UCB to develop and commercialize therapies that target alpha-synuclein in all indications around the world (Click here to read the press release).
That agreement involved the development NPT200-11 (initially re-named UCB-1332 – Source), which entered Phase I testing in healthy volunteers in 2015 (Click here to read a press release related to this and click here to read more about that trial).
That Phase I study was completed in March 2015, and the companies announced that “NPT200-11 was well tolerated in 55 volunteers after oral administration” (Source).
The plan was then for NPT200-11 to enter clinical testing in September 2016 for Multiple Systems Atrophy (or MSA – an alpha synuclein-associated orphan disease that is similar to Parkinson’s – click here to read a previous SoPD post on MSA). I have not been able to find any evidence that the MSA study was ever started.
And at this point, my understanding (and I am happy to be corrected here) is that NPT200-11 was re-named UCB0599. And in 2019, UCB initiated a US-based multicenter Phase Ib clinical trial of UCB0599 in Parkinson’s. 31 people with Parkinson’s (Hoehn-Yahr stage 1–3, aged 40–80 years) were recruited and they received two doses of either UCB0599 (n=21) or placebo (n=10) over four weeks (Click here to read the details of the trial and Click here to read the press release). This was the first time the drug was tested in people with PD.
The results of that study indicated that UCB0599 was safe and well tolerated in the study. There was little difference between the UCB0599- and placebo-treated groups in terms of treatment-emergent adverse events (headache being the most common event in both groups).
The investigators reported that “UCB0599 was generally well tolerated with no significant safety or tolerability concerns identified in a population of older individuals with Parkinson’s with age-related comorbidity” (Click here to read the abstract from the 2021 American Academy of Neurology virtual meeting where this data was presented).
The researchers concluded their presentation at the meeting by stating that the data supports “further investigation of UCB0599 for the potential to slow PD progression“.
Have they started any further investigations of UCB0599?
Yes, they have.
In December 2020, UCB initiated the Phase IIa “ORCHESTRA” trial, which is a double-blind, placebo-controlled, randomized, 18-month study to assess the safety and tolerability of UCB0599 in 300 people with Parkinson’s.
The study will also be looking for any evidence that UCB0599 is superior to placebo in terms of slowing disease progression over 12 and 18 months (the primary outcome of the trial is MDS-UPDRS Parts I-III sum score).
The study is now recruiting individuals with early stage Parkinson’s at research sites in the U.S., Canada, and the Netherlands and the study is scheduled to finish in October 2023 (Click here to read more about this trial).
And this is where Novartis are stepping into the picture. Apparently, they like what they have seen and they are keen to be part of the action. And it should be noted that this is a big step for Novartis as they have nothing in their current pipeline for PD.
One addition note of interest in this new partnership is UCB7853.
What is UCB7853?
UCB7853 is an anti-alpha synuclein monoclonal antibody. This is a completely different agent to UCB0599 – which is a small molecule.
Antibodies are Y-shaped proteins that your immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).
Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove the bound object. Each antibody targets a very specific structure, while ignoring everything else.
In this fashion, antibodies are a very powerful method of removing items from the body that are causing trouble or not wanted. And researchers have adapted this natural system for Parkinson’s using what are referred to as immunotherapy approaches.
Immunotherapy is a way of boosting the immune system of the body.
Currently, immunotherapy is being tested in Parkinson’s in two ways:
- Active immunisation – this approach involves the body’s immune system being encouraged to target the toxic form of alpha synuclein. The best example of this is a vaccine – a tiny fragment of the troublesome pathogen (such as the toxic form of alpha synuclein) is injected into the body before the body is attacked, which helps to build up the immune systems resistance to the pathogen (thus preventing the disease from occurring – click here to read a previous SoPD post about efforts to make a PD vaccine).
- Passive immunisation – this approach involves researchers designing antibodies themselves that specifically target a pathogen (such as the toxic form of alpha synuclein, while leaving the normal version of the protein alone). These artificially generated antibodies can then be injected into the body.

As an anti-alpha synuclein monoclonal antibody that is injected into the body, UCB7853 is a form of passive immunisation.
As part of their new partnership, Novartis has the right to “opt-in” to the global co-development of UCB7853 upon completion of a Phase 1 study currently being run by UCB. That Phase I study is being conducted in London (UK) and is expected to complete in 2023 (Click here to read more about this study).
What is the difference between small molecule inhibitors like UCB0599 and immunotherapy approaches like UCB7853?
To date, antibody-based immunotherapies have had a very had time getting into the brain and they are not able to enter cells. They are very large molecules.
And this is where small molecules have an advantage: they are tiny, they enter the brain, and are regularly taken up by cells. So small molecules are able to inhibit alpha synuclein aggregation inside of cells.
|
# # RECAP #2: A small biotech company developed an alpha synuclein aggregation inhibitor, which is now in clinical testing for Parkinson’s. UCB is clinically testing the drug – called UCB0599 – and they have partnered up with the Pharmaceutical company Novartis to develop the agent further. # # |
Interesting. Is UCB0599 the only small molecule inhibitor of alpha synuclein currently being clinically tested?
No.
There is also Anle138b, which has been developed by a small German biotech company called MODAG.

In addition to the MODAG/Anle138b trial, there is also a clinical trial being conducted in the UK called the ADepT-PD study.

Cure Parkinson’s is supporting a sub-study of this larger trial exploring the disease-modifying potential of nortriptyline.
What is nortriptyline?
There are different types of antidepressants, such as:
- Serotonin-noradrenaline re-uptake inhibitors (SNRIs), which block the reabsorption of both serotonin and another chemical called noradrenaline. Common examples of SNRIs include Duloxetine (‘Cymbalta’ and ‘Yentreve’) and Venlafaxine (‘Efexor’).
- Noradrenaline and specific serotonergic antidepressants (NASSAs), which are similar to SNRIs but generally prescribed to people who are unable to take SSRIs. A commonly prescribed NASSA is Mirtazapine (‘Zispin’).
- Tricyclic antidepressants, which are one of the oldest classes of anti-depressant drugs available – we will come back to these very shortly.
Nortriptyline is a tricyclic antidepressant.
What are tricyclic antidepressants?
Tricyclic antidepressants are a class of antidepressant medications that derive their name from their chemical structure: they contain three rings of atoms.
The chemical structure of the tricyclic antidepressant Imipramine. Source: Wikipedia
First discovered in the 1950s, in addition to depression tricyclic antidepressants are used for a variety of conditions, from ADHD to chronic pain. They have been used for just about everything during their time, including reduction of bed-wetting and prevention of migraines.
Interesting, so why is nortriptyline being tested for disease modification in Parkinson’s?
This is Professor Tim Collier:
He is the director of the Michigan State University Udall Center of Excellence.
A few years ago, Prof Collier and a research scientist in his lab named Dr Katrina Paumier observed something interesting about the biological mechanisms associated with the actions of tricyclic antidepressant drugs.
Dr Katrina Paumier. Source: Translationalscience.msu
They noted some previous research indicating that tricyclic antidepressants regulate pathways involved in cell survival (Click here and here to read some OPEN ACCESS examples). This led them to ask if the tricyclic antidepressants that are regularly used to treat Parkinson’s-associated depression could also possibly slow down the progression of the condition.
They decided to test this idea, which gave rise to this research report:
Title: Tricyclic antidepressants delay the need for dopaminergic therapy in early Parkinson’s disease.
Authors: Paumier KL, Siderowf AD, Auinger P, Oakes D, Madhavan L, Espay AJ, Revilla FJ, Collier TJ; Parkinson Study Group Genetics Epidemiology Working Group.
Journal: Mov Disord. 2012 Jun;27(7):880-7.
PMID: 22555881
In this study, the researchers used a large database compiled from 6 completed clinical trials (ELLDOPA, QE2, TEMPO, PRECEPT, FS1 and FS-TOO – all conducted by the Parkinson’s Study Group and the Neuroprotection Exploratory Trials in Parkinson’s Disease Project (NET-PD)) to examine whether antidepressant treatment could alter the course of Parkinson’s progression. This shifting of the disease course was determined by the amount of time that passed before initiating dopamine-based therapies in people recently diagnosed with Parkinson’s.
A total of 2064 subjects were included in this analysis, of which 451 were on an antidepressant at some time during the study. What the investigators found in through their analysis was that the time to dopaminergic therapy was shorter for those with baseline depression compared to those without depression, suggesting that the presence of mild depression in early Parkinson’s is associated with needing dopamine therapy earlier. And this finding applied to those individuals who were also taking an antidepressant.
But, when the researcher looked at the different classes of antidepressants being taken, they noticed something really interesting: the average time to needing dopamine therapy was longer for those on amitriptyline (compared to those subjects not taking antidepressants).
Amitriptyline is a tricyclic antidepressant.
Individuals taking tricyclics antidepressants (amitriptyline in particular) had a lower probability of initiating dopaminergic therapy within the first year of their study. This observation suggested to the researchers that treatment with tricyclic antidepressants may delay the need to initiate dopamine therapy for Parkinson’s.
So what did Prof Collier and his colleagues do next?
Their study suggested an effect, but it could not explain why this would be the case and so the researchers next turned their attention to better understanding what could be causing this delay in the need to initiate dopamine therapy.
And that effort resulted in this research report:

Authors: Paumier KL, Sortwell CE, Madhavan L, Terpstra B, Celano SL, Green JJ, Imus NM, Marckini N, Daley B, Steece-Collier K, Collier TJ.
Journal: Neuropsychopharmacology. 2015, 40(4):874-83.
PMID: 25267343 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers investigated whether long term treatment the tricyclic antidepressant – amitriptyline – would have any neuroprotective in a neurotoxin (6-OHDA) model of Parkinson’s. The investigators treated the animals for two weeks before giving the neurotoxin and they found that the treatment resulted in a significant rescue of the dopamine neurons in the brain and prevented the motor-related behavioural problems associated with the model. The researchers also noted that amitriptyline treatment increased the production of neurotrophic factors.
What are neurotrophic factors?
A neurotrophic factor (neurotrophic = Greek: neuron – nerve; trophikós – to feed) is a protein that is produced in the brain that helps to nourish the neurons and keep them alive. There are many different types of neurotrophic factors, but in this study the researchers were measuring just brain derived-neurotrophic factor (or BDNF) and Glial cell line-derived neurotrophic factor (GDNF) – both of which have been found to support dopamine neurons (one of the populations of cell badly affected in Parkinson’s).
Amitriptyline treatment resulted in an increase in BDNF in the substantia nigra region both before and during ongoing degeneration, suggesting it may contribute to neuroprotection observed in vivo. Amitriptyline treatment also resulted in an increase in GDNF levels but only before the neurotoxin was given (We have previously discussed GDNF a few times on this website – click here to read an example).
Nervous that pre-treatment of amitriptyline could be having an effect on dopamine processing, the investigators also looked at levels of the dopamine transporter (which is a protein on the surface of dopamine neurons that helps to reabsorb used dopamine back into the cell) in animals that had had long-term treatment with the anti-depressant (compared to untreated controls). They found no difference in dopamine transporter levels between the two groups, and so they concluded that the neuroprotective effect seen in their model was most likely due to the increase in BDNF levels.
This initial report was quickly followed up by a second study that supported these results:
Title: Tricyclic antidepressant treatment evokes regional changes in neurotrophic factors over time within the intact and degenerating nigrostriatal system.
Authors: Paumier KL, Sortwell CE, Madhavan L, Terpstra B, Daley BF, Collier TJ.
Journal: Exp Neurol. 2015 Apr;266:11-21.
PMID: 25681575 (This article is OPEN ACCESS if you would like to read it)
In this study, Prof Collier and colleagues conducted a time-course study to determine whether the tricyclic antidepressant amitriptyline could cause changes in the levels of neurotrophic factors in relevant brain regions in both normal rats and those used to model of Parkinson’s (intrastriatal injection of the neurotoxin 6OHDA).
The researchers found that after continuous treatment of amitriptyline to normal rats for 24 days, there was a significant increase in levels of both BDNF and GDNF in the substantia nigra region of the brain – where the dopamine neurons reside. In the rodents that were used in the modelling of Parkinson’s, there was a transient increase in these neurotrophic factors, which helped to reduce the progressive degeneration of dopamine neurons elicited by the neurotoxin.
All of these findings supported the investigators proposal that tricyclic antidepressants may have dual therapeutic value in Parkinson’s.
Interesting. But what does this have to do with nortriptyline and alpha synuclein inhibition?
Great question.
Following the publication of this neurotrophic study, the researchers in Michigan were contacted by Dr Peter Lansbury and Dr Craig Justman (from Lysosomal Therapeutics, Inc – a Boston-based biotech company that was taking a drug called LTI-291 to the clinic.

Dr Lansbury and Dr Justman also had experimental evidence that the tricyclic antidepressants amitriptyline and nortriptyline slowed the clustering (or aggregation) of alpha synuclein. They had no intension of following up this interesting result as it was outside of the company’s focus, but they thought that Prof Collier and his colleagues may be interested.
Prof Collier and his colleagues had also contacted Dr Lisa Lapidus at Michgan State University and asked her to test nortriptyline in her well characterised assay of anti-aggregation effects. She found that the tricyclic antidepressant (though she was not aware of what kind of compound she was testing) had potent anti-aggregation effects in her assay in a dose-related manner.
That’s validation, right?
The researchers expanded on these initial findings and published their results earlier this year:
Title: Nortriptyline inhibits aggregation and neurotoxicity of alpha-synuclein by enhancing reconfiguration of the monomeric form.
Authors: Collier TJ, Srivastava KR, Justman C, Grammatopoulous T, Hutter-Paier B, Prokesch M, Havas D, Rochet JC, Liu F, Jock K, de Oliveira P, Stirtz GL, Dettmer U, Sortwell CE, Feany MB, Lansbury P, Lapidus L, Paumier KL.
Journal: Neurobiol Dis. 2017 Oct;106:191-204.
PMID: 28711409
In addition to findings of Dr Lansbury, Dr Justman and Dr Lapidus described above (regarding the anti-aggregation properties of nortriptyline), the researchers also treated aggregation-proned cells in culture with nortriptyline and found reduced accumulation of alpha synuclein. They then tested nortriptyline on dopamine neurons that produced very high levels of a mutant form of alpha synuclein protein (A53T) which eventually kills the cells. By exposing these cells being grown in cell culture to nortriptyline, the researchers observed reductions in the number of cells dying (in a dose dependent manner).
Next the investigators turned their attention to the in vivo setting, and utilised both fly and mouse models of Parkinson’s to test nortriptyline. The flies that the researchers used have very high levels of a mutant form of alpha synuclein protein (A30P), which results in neurodegeneration. By feeding the flies with nortriptyline-supplemented food, the researchers reported a significant reduction in the amount of neurodegeneration observed. In mice that produced very high levels of human alpha synuclein protein, the investigators started treating the mice with nortriptyline daily for one month at 6 months of age. When they looked at the brains of these treated mice, they found that they had less alpha synuclein accumulation than the untreated group of mice.
Now, as if all of these experiments were not enough, the researchers decided to go yet further:

Authors: Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, Sandoval IM, Fleming S, Dirr E, Polinski NK, Trojanowski JQ, Lee VM, Sortwell CE.
Journal: Neurobiol Dis. 2015 Oct;82:185-199.
PMID: 26093169 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers injected rodents with alpha synuclein fibrils to model Parkinson’s.
In their previous study, the researcher injected rodents in a particular location of the brain with alpha synuclein fibrils, and then left the animals for up to 18 months to see what impact these proteins would have. They found cellular inclusions that had the characteristics of the features observed in the Parkinsonian brain (such as Lewy bodies). The rodents also demonstrated the loss of dopamine neurons. Thus, the investigators were excited about the possible use of these alpha synuclein fibrils to better model Parkinson’s and test new drugs… such as nortriptyline.
The investigators tested the ability of nortriptyline to reduce the effect of alpha synuclein fibrils to cause Parkinson’s-like damage. The alpha synuclein fibrils were injected into the brains of rodent, and two weeks later the rodents were randomly assigned to groups that received daily treatment of nortriptyline or saline. This treatment was conducted for 6 weeks, and at the end of that period, the saline-treated animals exhibited the expected accumulation of alpha synuclein in the brain, while the nortriptyline treated animals had consistently less aggregation.
In the image below, the alpha synuclein pre-formed fibrils (PFF) + saline treated animal has a lot more aggregated alpha synuclein (black dots diagonally across the image) than the PFF + nortriptyline (NOR) treated brain.
Source: CollierLab
The researchers also found that nortriptyline was directly binding to alpha synuclein and keeping it resistant to forming fibrils, thereby inhibiting pathological aggregation while also preserving its normal functioning. They concluded that nortriptyline, “with a long history of safety and efficacy, may provide multiple benefits” for Parkinson’s, “including amelioration of comorbid depression, modulation of important signaling pathways involved in cell survival and plasticity, reduced inflammation and interference with misfolding and aggregation of alpha synuclein: a multi-function compound to treat a complex disorder”.
Do you think I should start taking tricyclic antidepressant?
This is an obvious question to ask. And I know that I’m going to get a bunch of emails asking this exact question.
If I receive any emails of this nature, however, I will copy-and-paste in the following text:
“You will hopefully understand that I am very reluctant to give advice on such matters. This is for two reasons:
A. I am only a research scientist (not a clinician), and
B. Even if I was a clinician, it would be unethical for me to offer advice being unaware of the personal medical history/circumstances in each case”
Before considering any changes to one’s treatment regime, you really must have a thorough discussion with your neurologist or a clinician that is familiar with your medical history. I certainly won’t be dishing out advice.
And please appreciate that all of this research (except for the first epidemiological studies) has been conducted in cells grown in culture and laboratory animals.
Source: Irishtimes
All of this research makes Cure Parkinson’s supported sub-study of the ADepT-PD clinical trial rather interesting.
This over all study will involve 408 participants who will be randomly assigned nortriptyline, escitalopram or the placebo treatment, and they will then be followed for 1 year (Click here to read more about this).
This trial is currently recruiting – Click here if you are interested in learning more about potentially taking part in this study.
So what does it all mean?
Phew. Long post. Well done if you managed it all in one sitting – I owe you a beer. I’ll make this summary brief.
It is never a good time to have Parkinson’s, but it is a fascinating time for the research surrounding the condition. After the association of alpha synuclein with PD back in 1997, we may soon have answers to the question of whether reducing it can slow the progression of Parkinson’s.
With three different lines of clinical research exploring intracellular inhibition of the aggregation of the protein via small molecules, we will hopefully see some clarity on the role that this protein plays: Is it the villain or just the hapless fool caught holding the knife?
If found to be the villain, what will future treatment look like? How often would one need to take an inhibitor (once per day or once per week?)? Would it be taken in combination with an immunotherapy (dealing with synuclein inside and outside of the cell)? Fingers crossed it all goes well – these would be good problems to have.

Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The author of this post is an employee of the Cure Parkinson’s Trust, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from SoPD