|
# # # # At the start of each year, it is a useful practise to layout what is planned over the next 12 months. The events that are scheduled for the year to come, so that we can keep an eye out for them. Obviously, where 2021 will end actually is unpredictable, but an outline of what is scheduled over the next 365 days will hopefully provide us with a useful resource for helping to manage expectations. Here at the SoPD, we are primarily interested in disease modification for Parkinson’s. While there is a great deal of interesting research exploring the causes of the condition, the genetics and biology of the condition, novel symptomatic therapies, and other aspects of Parkinson’s, my primary focus is generally on the science seeking to slow, stop or reverse the condition. In this post, I will try to map out some of what is scheduled to occur in 2021 with regards to clinical research focused on disease modification for Parkinson’s. I will also note aspects of ongoing research where I will be hoping to see an update on progress. It will be an extremely (read: ridiculously) long post, but it will hopefully give readers a feel for what the landscape looks like for research focused on disease modification for Parkinson’s. # # # # |

Cartography is the study and practice of mapping things out. It has been used for centuries to provide graphic representations of what stuff looks like to help us to better understand things.
The word cartography comes from the Greek words χάρτης or chartēs (meaning “papyrus, sheet of paper”) and γράφειν or graphein (meaning “to write”).
According to Wikipedia, the fundamental objectives of traditional cartography are to:
- Set the map’s agenda and select traits of the object to be mapped.
- Represent the terrain of the mapped object on flat media.
- Eliminate characteristics of the mapped object that are not relevant to the map’s purpose.
- Reduce the complexity of the characteristics that will be mapped.
- Orchestrate the elements of the map to best convey its message to its audience.
At the start of each year, the SoPD publishes a horizon scanning post where we take a cartography-like approach towards laying out the landscape of clinical research focused on disease modification for Parkinson’s for the next 12 months.

We try to “set the agenda” and “select traits” to look out for in 2021. We also try to “represent the terrain” and “reduce the complexity of the characteristics” (well,… at least we will try to!) in a manner that will “best convey” to the reader what the next 12 months may look like.
All of this is in an effort in better managing expectations about some of the research results that are coming down the pipe.
To be clear, this post is NOT intended to be an exercise in the reading of tea leaves (no predictions will be made here). Nor is this a definitive or exhaustive guide of what the next year holds for disease modification research (if you see anything important that I have missed – please contact me or leave a comment below).
Readers should be aware that there is still a long way to go before any of these potential therapies will be made available in the clinical setting. And it should certainly not be assumed that any of the indvidual treatments mentioned below are going to be silver bullets or magical elixirs that are going to “cure” the condition. As I have discussed many times on this website, a “curative therapy” for Parkinson’s is going to require three core components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of restorative therapy
Now, the bad news is (as far as I am aware) there is no single treatment currently available (or being tested) that can do all three of these things. By this I mean that there is no disease halting mechanism therapy that can also replace lost brain cells. Nor is there a restorative therapy that stop the progression of the condition.
That last paragraph can obviously be read as bad news, but it shouldn’t.
Let me explain:
A curative therapy for Parkinson’s is going to need to be personalised to each individual, with varying levels of each of the three component listed above. It will be a multi-modal approach, designed to best fit each individual’s needs.

By this I mean, there is a great deal of heterogeneity (or variability) between individuals with regards to their Parkinson’s symptoms and the amount of time that they have had the condition. No two cases of Parkinson’s are the same. Some folks are more tremor dominant, while others do not experience tremor at all. Likewise, some individuals have only just been diagnosed, while others have lived with the condition for many years.
As a result, the treatment needs of each individual will be different, and thus what we will require is differing amounts of each component for each individual. By this I mean, that someone who has only just been diagnosed may only need the disease halting mechanism component, while someone who has had the condition for many years will need different amounts of all three components (depending on their situation).
Now the good news is that there is considerable clinical research currently being conducted on each of these three components. And we will now explore what research is happening in each of these components and discuss what is scheduled for 2021.
|
SPEACIAL NOTE: Before we start, this website is the personal blog of the deputy director of research at The Cure Parkinson’s Trust. The Trust is a UK registered charity which is an international supporter of many of the clinical trials mentioned in this post. To avoid any bias and for the purposes of full disclosure, where appropriate I will note the Trust’s involvement. In addition, I would like to thank Parkinson’s research advocates Sue Buff, Gary Rafaloff and Kevin McFarthing for the efforts they put into maintaining their wonderful databases of Parkinson’s clinical trials (Sue maintains the PDTrialTracker website, while Kevin keeps the “Hope list“). This post would not be possible without those amazing resources. And they produced a magnificent report last year on the drug development pipeline for Parkinson’s, which provides a broader review and analysis than is provided here – it is recommended reading for those interested (Click here to read that report). |
In previous years, this annual “Road ahead post” has read like an endless shopping list. This year to save the reader’s sanity a contents index is being provided here. Hopefully when you click on the section that interests you in this index, you will jump to that section. To come back to the index, simply hit the ‘return’ button on your browser.
THE INDEX
COMPONENT #1: A disease halting mechanism
- The direct approach
- The direct approach: Alpha synuclein
- Passive immunotherapy approaches for alpha synuclein
- Active (vaccine) immunotherapy approaches for alpha synuclein
- Small molecule approaches targeting alpha synuclein
- The direct approach: LRRK2
- The direct approach: GBA
- The direct approach: Mitochondria
- The direct approach: Additionals
- The indirect approach
- The indirect approach:Autophagy
- The indirect approach: Inflammation
- The indirect approach: Gastrointestinal system
- The indirect approach: Iron chelation
COMPONENT #2: A neuroprotective agent
- GLP-1 agonists
- Neurotrophic factors
- Glial cell-derived neurotrophic factor (or GDNF)
- Cerebral dopamine neurotrophic factor (or CDNF)
- Neuroprotective approach: Additional
COMPONENT #3: Some form of restorative therapy
I hope this helps with navigating this long post (you have been warned: it’s a doozie!). Let’s now consider the first component of any curative therapy for Parkinson’s:
COMPONENT #1. A disease halting mechanism
Parkinson’s is a progressive neurodegenerative condition. Thus, the first and most critical component of any ‘cure’ for Parkinson’s involves a treatment that will slow down or halt the progression of the condition.
This can be done either directly or indirectly.
The direct approach
The direct approach involves treatments that specifically target the underlying biology of the condition.
A direct approach in halting Parkinson’s, however, requires a fundamental understanding of how the condition is actually progressing. And if we are honest, we are not there yet – we still do not have a solid grasp of how Parkinson’s progresses over time. In addition, this may vary between individuals. It is gradually being agreed that rather than being a single ‘disease’, Parkinson’s may actually be a ‘syndrome’ – that is, a collection of conditions that share similar symptoms.
We do, however, have some solid theories as to what is happening, and there are numerous clinical trials focused on attempts at “direct approaches” to halting Parkinson’s. For example, there is a protein called alpha synuclein which is known to build up in many cases of Parkinson’s.

This build up of alpha synuclein protein results in the appearance of structures called Lewy bodies in the brains of people with Parkinson’s.

It is believed that alpha synuclein might be passed from cell to cell, ‘seeding’ the condition in each cell as it goes, and thus, it may underly the slow progressve nature of Parkinson’s. Researchers have proposed that targeting this protein could represent a means of slowing/halting the progression of Parkinson’s.
The direct approach: Alpha synuclein
One of the direct approaches being employed against alpha synuclein is a method called immunotherapy.
Immunotherapy involves boosting the body’s immune system to target specific toxic agents in the body. In the case of Parkinson’s, this approach is primarily being focused on different forms of alpha synuclein.
Antibodies. Source: Astrazeneca
The immunotherapy approach uses antibodies, which are Y-shaped proteins that act like alert flags for the immune system. Once enough antibodies bind to a particular object, the immune system will dispose of it. Antibodies target very specific structures, while ignoring everything else.
In Parkinson’s, the immunotherapy approaches are primarily involving antibodies that target the alpha synuclein protein. By tagging the alpha synuclein as it is being passed from one cell to another, and allowing the immune system to remove it, researchers hope to slow down the progression of Parkinson’s.
Immunotherapy can be conducted in two ways:
- The body’s immune system can be encouraged to develop its own antibodies that target the toxic form of alpha synuclein (using active immunisation in the form of a vaccine); or
- Researchers can design antibodies themselves that specifically target the toxic form of alpha synuclein (while leaving the normal version of the protein alone), and then inject those antibodies into the body (passive immunisation)

Passive immunotherapy approaches for alpha synuclein:
There are now numerous biotech firms testing passive immunotherapy approaches in the clinic for Parkinson’s, but in reality there are two main studies in the immunotherapy for Parkinson’s that everyone has their eyes on (simply because of their advanced progress in the clinical trial process).
In 2020, the results of the PASADENA study were announced. This study is a Phase II clinical trial of an alpha synuclein targeting immunotherapy (called Prasinezumab – formerly called RO7046015 & PRX002) being conducted by the pharmaceutical company Roche and biotech firm Prothena Biosciences (Click here to read a SoPD post about this).
The companies announced that the trial had not met its primary endpoint (a predetermined measure of efficacy), but that prasinezumab “showed signals of efficacy” , and importantly: “These signals were observed on multiple prespecified secondary and exploratory clinical endpoints“.
Specifically, when the researchers looked at just the motor scores of the participants (UPDRS Part III), there was evidence of a slower progression in the participants treated with prasinezumab than those treated with placebo (remember, these individuals were all blind to their treatment):

Roche and Prothena have concluded that their “findings support the potential of prasinezumab to slow underlying disease pathophysiology and clinical decline in patients with PD. Further investigations are warranted” (Source) and the two companies are continuing to follow up participant in the current Phase II study, and planning to launch a Phase IIb clinical trial in patients with recently diagnosed Parkinson’s in early 2021 (Source).
It is very important to remember that these observations are based on post hoc analysis (that is, after-the-fact re-analysis of the trial data) and they should not be considered as evidence of efficacy. But it would be encouraging if additional immunotherapy trials show signals that also warrant further investigation.
Speaking of which…
The second main immunotherapy study is the SPARK study being conducted by the Pharmaceutical company Biogen.


In addtion to the Pasadena and SPARK studies, there are a large number of other biotech companies developing immunotherapy programmes for Parkinson’s, including:
- Astrazeneca‘s immunotherapy treatment called MEDI1341 (being developed with Takeda Pharmaceutical) is currently in Phase I safety testing in healthy volunteers. This study is scheduled to finish in April 2021 – thus we should get some news/update regarding this treatment in 2021 (Click here to read more about that study). In addition, in June 2020, Astra registered a second Phase I study assessing multiple ascending doses of MEDI1341 in people with Parkinson’s. That new study is scheduled to complete in July 2022 (Click here to read more about this trial).
- Lundbeck‘s immunotherapy treatment called Lu AF82422 (which is being developed in collaboration with Genmab) was in Phase I safety testing in both healthy volunteers and people with Parkinson’s during 2020 and it was scheduled to finish in December 2020 (Click here to read more about this). We are hoping to get an update regarding the progress of Lu AF82422 in 2021 and news of potential further development.
- In March 2020, the pharmaceutical company AbbVie started a multicenter, placebo-controlled Phase I study of their immunotherapy treatment called BAN0805/ABBV-0805 (Click here to read more about this – this immunotherapy approach is being developed in collaboration with BioArctic Neuroscience). In June 2020, however, the Phase I study was withdrawn and BioArctic announced that a detailed plan to accelerate ABBV-0805 into a Phase II Proof of Concept study in Parkinson’s is now being prepared by AbbVie (Source). We look forward to learning more about this in 2021. It is interesting to note that these two companies have two additional alpha synuclein-targeting immunotherapy treatments called PD1601 & PD1602 which are also in development.
|
Preclinical developments in passive immunotherapies to look out for in 2021: In addition to the companies with clinical programmes there are other biotech firms developing antibody-based therapies targetting alpha synuclein for Parkinson’s. These include:
There are also several companies developing gene therapy-based immunotherapy approaches focused on alpha synuclein, including:
|
Now you may recall that we mentioned two types of immunotherapy above – passive and active (‘passive’ requiring regular injections of antibodies, while ‘active’ enables the immune system to produce the antibodies, requiring less treatments). The clinical trials we have discussed above is passive immunotherapy.
In addition to these passive immunotherapy treatments, there are also two biotech companies that are testing active immunotherapy treatment in Parkinson’s. These are vaccines for Parkinson’s, which targets the toxic form of alpha synuclein.
Active (vaccine) immunotherapy approaches for alpha synuclein:
The company with the most advanced vaccine program is called AFFiRiS.
They have been clinically testing a vaccine treatment called ‘AFFITOPE® PD01A’ and in July 2020, the results of their Phase I studies were published in the journal Lancet Neurology. (Click here to read more about this).
While it is important to remember that this trial was an ‘open label’ study (meaning that all of the participants knew what they were being treated with and a placebo response could have been at play), the results were rather interesting. Firstly that the treatment is safe and well tolerated in the participants, and the vaccine caused the immune system to start producing alpha synuclein targeting antibodies. In addition, by 26 weeks into the study, the researchers observed a 51% reduction in cerebrospinal fluid levels of aggregated alpha synuclein.
Regarding some basic assessments of disease progression, the researchers wrote in their report that: “DAT-SPECT examinations did not show statistically significant changes up to 91 weeks in study 1. MDS-UPDRS part 3 scores were generally stable across the studies”.
This sentence suggests that the researcher did not see any brain imaging or clinical evidence of disease progression. But again, this was an open label study, and a larger, double blinded evaluation of the PD01A treatment is now required.
In January 2020, AFFiRiS announced that based on feedback from the US FDA, they could proceed with preparations for a Phase II clinical trial. They planned to initiation that study in the US and Europe in the second half of 2020 (click here to read more about this), but COVID-19 may have delayed those plans as no announcement of the new trial starting has been made. We look forward to news of this in 2021.
A second company developing a vaccine against alpha synuclein is Vaxxinity (formerly known as “United Neuroscience”).
This biotech company is focused on developing a novel class of vaccines that are fully synthetic (they call them ‘endobody vaccines‘) and can train the body to treat/prevent neurological condtions. They are currently conducting a Phase I safety/tolerability trial of UB-312 in healthy volunteers and in participants with Parkinson’s. The results of this study are scheduled for June 2022 (Click here to read more about this trial).
|
Preclinical developments in active immunotherapies to look out for in 2021: As with the passive immunotherapies, there are also biotech companies with active immunotherapies (vaccines) in preclinical development. Capo Therapeutics is a small biotech with alpha synuclein targetting vaccines (AV-1947D, AV-1948D, AV-1949D, AV-1950R, and AV-1950D) in development. |
One of the acknowledged limitation of the immunotherapy approaches, is the low amount of antibody actually accessing the brain (though Denali Therapeutics & Biogen are collaboratively developing technology to improve this situation – click here to read a previous SoPD post about this).
In the most of the immunotherapy trials to date, only 1-3% of the treatment in the blood is actually getting into the brain. This is due to a protective membrane surrounding our brains, called the blood brain barrier, which limits entry of most drugs/proteins.
These limited amounts of immunotherapy treatment still allowed for the clearance of the targeted protein (beta amyloid in the case of the Alzheimer’s trials) so it can be assumed that it should also be enough to be able to reduce levels of extracellular alpha synuclein in the Parkinson’s immunotherapy clinical trials.
But these immunotherapy trials will also have limited ability to affect alpha synuclein within cells (remember, they are tagging and grabbing the protein as it is being passed between cells). This situation has led a growing number of biotech companies to develop small molecules that can enter and target alpha synuclein inside of cells.
Small molecule approaches targeting alpha synuclein:
One example of a small molecule that is targeting alpha synuclein is a drug called NPT520-34 which is being developed by a biotech firm called NeuaroPore Therapies.
They completed Phase I clinically testing of NPT520-34 in healthy individuals in September 2019 (Click here to read more about this trial). In January 2020, the company announced that “The results of this study support moving forward to a safety study in patients. Our team is currently evaluating the optimal study design and patient population for the next study” (click here to read that press release). COVID-19 has obviously delayed progress, but we will be looking out for news of future development in 2021.
NeuroPore Therapies have also out-licensed another small molecule alpha synuclein inhibitor called NPT200-11 to the pharma company UCB.
This drug has also been Phase I tested (Click here to read more about that trial). The study was completed (Click here to read the press release), and we look forward to learning more about the future development of this drug in 2021.
In 2020, we learnt that the biotech firm Annovis (formerly QR Pharma) had initiated clinical testing of their alpha synuclein targeting molecule called ANVS-401 (also known as Posiphen) in individuals with Parkinson’s or Alzheimer’s (Click here to read more about this).

Another experimental small molecule targeting alpha synuclein, called ENT-01, is currently being clinically tested a company called Enterin Inc.
Between 2017-2018, the company conducted the RASMET study, which was a Phase I safety clinical trial of ENT-01 (Click here for the details about this trial and click here to read a SoPD post on this topic). The issue with ENT-01 compared to other molecules targeting alpha synuclein is that it does not cross the blood brain barrier. Thus, Enterin are focusing their clinical trial on Parkinson’s-associated constipation – can this drug reduce alpha synuclein aggregation in the gut and alleviate complaints like constipation.
The results of the Phase I RASMET study have been published (Click here to read them and click here to read the press release), and the company is currently conducting a Phase IIa ‘KARMET’ clinical study of ENT-01, which is scheduled to finish in September 2021 (Click here to read more about this study).
In addition to ENT-01 which does not cross the blood brain barrier, Enterin has a strong patent position around another molecule called Trodusquemine which does get into the brain. It is very similar to ENT-01 (also known as squalamine):

The assumption is that the company is conducting preclinical evaluations of trodusquemine (or a derivative of it) with the goal of taking it forward for Phase I clinical testing. We hope to learn more about this in 2021.
One of the more novel alpha synuclein-targting approaches for Parkinson’s is being clinically tested by the biotech company Yumanity.
This company is developing Stearoyl CoA desaturase inhibitors – these are a class of drugs that have been reported in preclinical research to reduce alpha synuclein-associated toxicity (Click here to read a SoPD post on this topic). In October 2019, Yumanity initiated Phase I clinical testing their first drug, YTX-7739, in 48 healthy individuals (Click here to read the press release and click here to read more about that trial), and there is another Phase I trial underway exploring multiple doses (Click here to read more about this trial). These trials are scheduled to finish in late 2019, but COVID-19 may have slowed progress here. Hopefully we will learn more in 2021.
A topic of great interest to many readers of the SoPD has been the sweetner Mannitol, which – after some interesting preclinical results – was crowd sourced into a patient-led online study by a group called Clinicrowd.
The results of that online study have been published (Click here to read a previous SoPD post about this topic), and have stimulated a Phase I clinical trial in Israel (Click here to read more about that trial). That trial was scheduled to finish in December 2020 so perhaps we will see the results of that study in 2021.
And finally a new entrant to the small molecule inhibitors of alpha synuclein field is Anle138b which is being developed by the biotech firm MODAG (Click here to read a previous SoPD post about this topic).

A lot of alpha synuclein trials finishing in 2021.
|
Preclinical developments in alpha synuclein-targeted small molecules to watch out for in 2021: Novel molecules:
Gene therapy approaches:
PROTAC(-like) compounds: Proteolysis targeting chimera (or PROTAC) technology is a system of targeting and degrading intracellular proteins. Companies exploring alpha synuclein-targeted PROTACs (or PROTAC-like approaches) include Arvinas (source), C4 Therapeutics (Source), and Primary Petides (Source). |
Not all of the direct approaches for slowing the progression of Parkinson’s involve targeting alpha synuclein protein. In fact, there are some cases of Parkinson’s that do not involve any accumulation of alpha synuclein at all. For example, in many cases of LRRK2-associated Parkinson’s, postmortem analysis has indicated that there is very limited alpha synuclein.
The direct approach: LRRK2
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) is a Parkinson’s-associated protein, which becomes hyperactive in some cases of the condition. This over-active form of the protein is believed to be associated with the neurodegeneration seen in these cases of Parkinson’s.
To counter the over-active form of this protein in the carefully balanced environment of a cell, researchers have been developing inhibitors of this protein. The hope is that by inhibiting LRRK2, function in the cell will be able to return to normal (or more manageable levels) which will make cells healthier. By doing this we may be able to slow down/halt the cell death and stablise the course of Parkinson’s.
Leading the pack in the race to develop LRRK2 inhibitors is a biotech firm called Denali Therapeutics.
Set up by a group of ex-Genentech scientists, Denali has been clinically testing two LRRK2 inhibitors: DNL-151 and DNL-201. In 2020, the company announced that they have finished Phase I testing of these drugs and they signed an agreement with the pharmaceutical company Biogen to co-develop and co-commercialise DNL151 (also being called BIIB122) as the lead LRRK2 inhibitor (Click here to read a previous SoPD post about this).
In July of 2020, the US FDA cleared an Investigational New Drug (IND) application for DNL151 enabling an expansion of Denali’s clinical trial program. Biogen and Denali are now working on finalising plans for two separate late stage (Phase II/III?) clinical trials in Parkinson’s:
- One study will be in patients with a LRRK2 genetic variant that causes hyperactivity of the protein, and
- The second study will involve participants with sporadic Parkinson’s (not associated with any genetic variant).
Enrollment for these trials is expected to commence in 2021. Denali has set up a website (EngageParkinson’s) for anyone seeking to learn more.
In 2021, we will also be looking for updates from Denali regarding their collaboration with gene therapy company, SIRION Biotech.

Another interesting note from the Biogen/Denali collaboration is where Biogen’s own LRRK2 inhibitor programme sits.

The companies have been working on BIIB094 – an antisense oligonucleotide targetting LRRK2. Antisense oligonucleotides are a method of inhibiting RNA rather than proteins – this means that this drug blocks LRRK2 RNA rather than the subsequent protein (Click here to read a previous SoPD post about this approach).
A Phase I clinical trial of BIIB094 was registered in late 2019. Called the “REASON study”, it involves 82 participants being recruited from 15 research centers in North America, Spain, Norway, the U.K., and Israel. The study is scheduled to complete until December 2022 (Click here to read more about this study).
|
Additional developments to look out for in 2021 regarding LRRK2-targeting agents: Small molecule inhibitors:
Antisense oligonucleotide approaches for LRRK2:
RNA editing approaches for LRRK2:
|
Another direct approach for slowing the progression of Parkinson’s is focused on an enzyme that is involved with the waste disposal/recycling system of cells:
The direct approach: GBA
Genetic variants in the GBA gene are among the most common risk factors for Parkinson’s. The GBA gene provides the instructions for producing an enzyme called Glucocerebrosidase (or GCase). This enzyme helps to break down glucocerebroside (into glucose and ceramide) in the lysosome (Click here to read a previous SoPD post about this).
In people with GBA genetic variants, it is believed that the GCase enzyme is not functioning correctly which results in aggregation of alpha synuclein protein and cell death. These individuals typically have an earlier onset of PD and a faster progression (although this can vary considerably between cases).
As a result of the association between GBA and Parkinson’s, a great deal of research has been conducted on the biology of this particular pathway, with multiple clinical trial programmes now testing GBA-based therapies.
In 2020, we saw the results of the “Ambroxol in Disease Modification in Parkinson Disease” (or AIM-PD) clinical trial clinical trial (Click here to read a SoPD post on these results).
Ambroxol. Source: Skinflint
This trial was supported by the Cure Parkinson’s Trust, and partners: the Van Andel Research Institute (USA) and the John Black Charitable Foundation.

The results of the AIM-PD study indicated that ambroxol was able to elevate levels of GCase in the brains of the participants:

A larger, longer clinical trial is now in development to evaluate the efficacy of ambroxol in Parkinson’s. We look forward to an update regarding this in 2021.
There is also a second Ambroxol study, which is being conducted in London, Canada. This is a phase II, 52 week trial of Ambroxol in 75 people with Parkinson’s Disease Dementia (Click here to read more about this trial). In this randomised, double blind study, two doses of Ambroxol were tested – a high dose (1050 mg) and a low dose (525 mg) – as well as a placebo treated group. This study is scheduled to finish in December 2021.
Another GBA-related experimental therapy is a drug called Venglustat (formerly known as GZ/SAR402671 & Ibiglustat) which is being conducted by the biotech company Sanofi Genzyme.
This drug is being tested in a clinical trial called “MOVES-PD”, which is a phase II clinical study that will be involve in two parts (Click here to read more about the trial):
- A dose escalation study to determine safety in early-stage GBA-associated Parkinson’s.
- A randomised, double blind study of efficacy of Venglustat, as compared to placebo in early-stage GBA-associated Parkinson’s
The preclinical results of this treatment approach looked promising (Click here to read some of the research on this), and the results of this clinical study are scheduled to be reported in 2021.
The results of the first part of the MOVES-PD study were presented at the Alzheimer’s/Parkinson’s 2019 meeting and Movement Disorder Society meeting in 2019, and they indicated that Venglustat is safe and well tolerated. We look forward to seeing the results of the study this year.
In 2020, gene therapy firm Prevail Therapeutics was acquired by the pharmaceutical company Eli Lilly (Click here to read about this).
This company is conducting the “PROPEL” trial in GBA-associated Parkinson’s with the aim of introducing a normal version of the GBA gene into the brain (via AAV9 viral vectors; the treatment is called PR001), allowing the cells to correct any lysosomal disfunction (Click here to read more about this trial). This trial does not complete until 2027, but we were expecting an initial update on progress with this trial at the end of 2020, which may have been delayed due to COVID and the acquisition. Looking forward to learning more in 2021.
Another company developing a GBA-based gene therapy approach is AVROBIO.

Another GBA-associated Parkinson’s clinical programme is being developed by PTC Therapeutics.
The company is currently conducting a Phase I clinical trial evaluating PTC857 in healthy volunteers. PTC857 is an inhibitor of 15-Lipoxygenase – this is an enzyme that is a key regulator of the oxidative stress, protein aggregation and inflammation response pathways. The company says that PTC857 is being developed for GBA-associated Parkinson’s (Source). The Phase I study does not appear to be registered so limited information is currently available, but given that it is a Phase I study I am assuming that this study should complete in 2021.
In 2021, we will also be watching out for the initiation of a new clinical trial for a drug called ESB1609, which is being developed by E-scape Bio (which also has a LRRK2 inhibitor programme mentioned above).

|
Additional developments to look out for in 2021 regarding GBA-targeting agents:
|
In addition to alpha synuclein, LRRK2, and GBA there are a number of other Parkinson’s associated proteins and pathways that believed to be playing a ‘direct’ role in the progression of Parkinson’s. Many of these revolve around the activity of mitochondria.
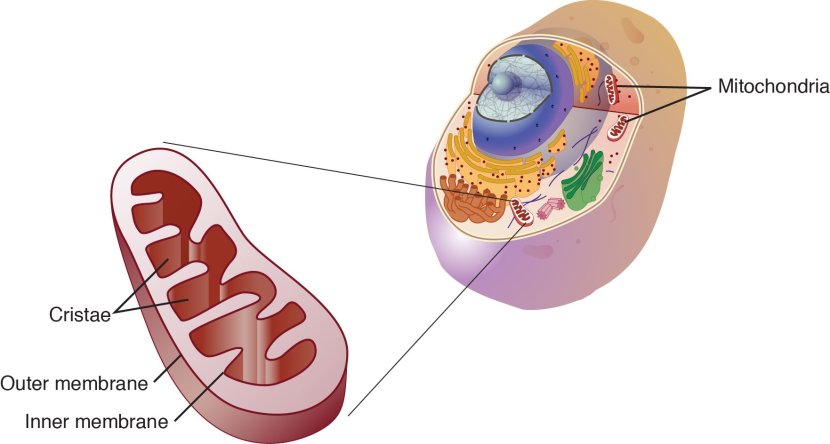
The direct approach: Mitochondria
Mitochondria are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
Mitochondria and their location in the cell. Source: NCBI
When mitochondria are old or damaged, they will start to release messenger proteins to alert the cell of their state. This will initiate a process of removing/disposing of the affected mitochondria – that process is called mitophagy. If too many mitochondria start excreting messenger proteins, however, the cell will become overwhelmed and die.
Mitochondrial dysfunction has long been associated with Parkinson’s. In addition, genetic mutations in several genes involved with the process of removing old/damaged mitochondria (mitophagy) have been associated with a higher risk of developing Parkinson’s (and this is why “mitochondria” are being considered a “direct approach” to component 1).
As a result, researchers have been developing therapies that a focused on improving mitochondrial function. By doing this, the theory is that the mitochondria will be healthier and able to better support the cell. Healthier cells will hopefully lead to a slower progression of Parkinson’s.

In February 2019, a clinical trial was initiated to evaluate UDCA (aka Ursodeoxycholic acid or ursodiol) in Parkinson’s. UDCA is a clinically-available medication for the treatment of gallstones and liver disease, and it is being repurposed for Parkinson’s based on preclinical research suggesting that it has beneficial effects on mitochondrial function. This property led to improvements in models of Parkinson’s, which resulted in the development of the “UP” study (“UDCA in Parkinson’s” study – Click here to read a SoPD post on the topic).
The Cure Parkinson’s Trust is a supporter of the UP study.
The study – involving 30 participants who are less than 3 years since diagnosis – is a Phase II, placebo controlled, double blind, randomised clinical trial, which was assessing the safety and tolerability of 30 mg/kg daily dosing of Ursodeoxycholic Acid (UDCA) in Parkinson’s. The study was completed in 2020, and we will find out the results in 2021.
There was a second clinical trial of UDCA in 2020, which was conducted at the University of Minnesota.
It was a Phase I open label study was designed to assess the safety/tolerability of increasing doses of UDCA (Click here to read more about this). That study published its results in 2020, and UDCA was found to be safe and well tolerated at the doses used (Click here to read a SoPD post on this topic). It will be interesting to learn of any plans for future clinical development of UDCA in 2021.
In 2021, we also hope to learn of future plans for another mitochondrial-targeted drug called CNM-Au8, which is being developed by a company called Clene Nanomedicine.

REPAIR-PD was a single-center open label pilot study to evaluate the safety, pharmacokinetics, and pharmacodynamics of CNM-Au8 in six individuals with Parkinson’s following 12 weeks of once daily treatment (Click here to read more about this trial). The results of the study demonstrated significant CNS target engagement of CNM-Au8, and indicate catalytic bioenergetic improvements (Click here to read more about this). It will be encouraging to hear news of this experimental agent move into a large, double-blind trial in 2021 (it is currently being tested in large ALS and MS trials).
South Korean firm Kainos Medicine completed a Phase I clinical study in 2019 evaluating their Parkinson’s candidate, KM-819 – a small molecule inhibitor for FAF1.

In September 2019, a research report was published that indicated that the prostatic hyperplasia and hypertension drug, Terazosin, had beneficial effects in models of Parkinson’s (Click here to read an SoPD post on this topic).

A clinical trial of Nicotinamide Riboside (a form of Vitamin B3) has started in Norway – it is called the ‘NOPARK’ Study. Nicotinamide Riboside is an important component in energy production and mitochondrial function – we have previously discussed the biology of Nicotinamide Riboside (Click here to read that SoPD post).

Another mitochondrial-targeting agent that I am hoping to hear news about in 2021 is EPI-589 (aka BioE-589) which is being developed by PTC Therapeutics (formerly BioElectron).
Preclinical data indicates that this drug helps to boost mitochondrial function. The company have conducted a Phase II open label, safety trial for the evaluation of EPI-589 in people with early onset genetic forms of Parkinson’s and also idiopathic Parkinson’s (Click here to learn more about this trial). And PTC had been planning further development – I’m assuming COVID delayed plans.
And this agent is interesting given the announcement in late 2018 of positive results for an open label Phase II clinical trial of EPI-589 in motor neurone disease/ALS. That study was assessing safety, tolerability, and disease biomarker effect, and the results “provide a strong rationale for the continued development of EPI-589” in ALS (Click here and here to read more about this and click here for the details of that study).
Another compound that has exhibited interesting results in ALS is CuATSM which is being developed by Collaborative Medicinal Development Pty (Click here to read more about the ALS result).
CuATSM is a highly effective scavenger of a chemical in our bodies called ONOO, which can be very toxic. In addition, there is evidence that the drug also blocks the aggregation of alpha synuclein and has beneficial effects in models of Parkinson’s (Click here to read an example).
In 2019, we learnt the results of a small Phase I clinical trial of CuATSM in Parkinson’s. The study found that 24 weeks of treatment with the drug was well tolerated, and the participants experienced some improvements in their symptoms (Click here to read more about this). This was an open label study, and we are still waiting to see the results published. But it would be again encouraging to see progress on a larger, double blinded, placebo-controlled study started in 2021.
Yet another company that recently announced a positive result in ALS is Amylyx.

|
Preclinical developments in mitochondrial research to look out for in 2021: Mitochondrial focused research programmes:
PINK & PARKIN targeting agents:
Deubiquitinating (DUB) enzyme inhibitors: Deubiquitinating enzyme make the removal of damaged mitochondria more difficult. Inhibiting them is viewed as a means of stimulating mitophagy (Click here to read a SoPD post on this topic).
|
In addition to the categorised direct approaches listed above, there are a number of new direct methods that are being explored and which deserve mention here as things to look out for in 2021, so we will add another category here called:
The direct approach: Additionals
The category refers to therapies in development that explore novel mechanisms of action associated with the underlying biology of Parkinson’s.
In 2020, a small biotech firm called CuraSen announced that they had dosed the first volunteer in their Phase I clinical trial of a selective adrenoceptor modulator called CST-2032 (Source).

The study will include approximately 70 healthy volunteers and individuals with different types of neurodegenerative diseases, including Parkinson’s. It is taking place in both New Zealand (32 pariticipants) and Belgium (Click here to read more about clinical trial and click here to read a SoPD post about this topic). If all goes well, Curasen will be looking to initiate Phase II testing in 2021.
In 2021, we are hoping to see more clinical trials starting focused on novel mechanisms of action that take a ‘direct approach’ towards slowing, stopping or reversing Parkinson’s.
|
Preclinical developments in “The direct approach: Additionals” category to look out for in 2021:
|
This is where activities currently lie with regards to (what I have described as) the direct approach to slowing or halting Parkinson’s progression. As I wrote above, the direct approach involves treatments that specifically target the underlying biology of the condition. This suggests that a particular gene or biological pathway is associated with Parkinson’s.
We will now shift our attention to the:
The indirect approach
While the direct approach to halting disease progression is focused on what we know about the underlying biology of Parkinson’s, indirect approach does not.
In our discussion here, an indirect approach is one that does not necessarily target a protein or biological pathway that is directly associated with Parkinson’s, but rather it attempts to slow progression by improving the overall health of affected cells, and allowing them to function better in the face of whatever is driving Parkinson’s.
One way we can improve the health of cells (and potentially slow the progression of Parkinson’s) is to enhance their ability to clear (or dispose of) old and potentially toxic proteins. This approach generally involves boosting the waste disposal systems of the cell – in this manner, the cells can break down and dispose of excess proteins (like alpha synuclein) inside the cell before they have a chance to builds up and becomes toxic.

A great deal of Parkinson’s research has focused on enhancing the cellular waste disposal/recycling process, which is referred to as autophagy.
The indirect approach:Autophagy
Helping cells to clean themselves up by boosting waste disposal systems, we will hopefully make the cells healthier and function better. And by limiting the build up of proteins – like alpha synuclein – these experimental therapies may help to slow down the progression of Parkinson’s.
As you shall see below, there are numerous clinical trials currently testing different therapies attempting to boost the autophagy process. One ‘autophagy boosting’ approach to slowing Parkinson’s involves a class of drugs called c-Abl inhibitors.
These molecules started life as cancer drugs, but they are now being re-purposed for Parkinson’s.
c-Abl is a protein that becomes activated in cells that are stressed and inhibiting it can boost autophagy. Multiple independent labs have demonstrated that this is a worthy target for Parkinson’s (Click here to read a review on this topic).
The first c-Abl inhibitor to be clinically tested in Parkinson’s was Nilotinib.

Following evidence suggesting beneficial effects in models of Parkinson’s and a small open label Phase I pilot study (Click here to read an old SoPD post about this topic), two large double-blind Phase II clinical trials were initiated: PD Nilotinib and NiloPD.
PD Nilotinib, was conducted at Georgetown University in Washington DC (Click here for the more details about this study), and in late 2019 the investigators reported that the drug was safe at lower doses, but “no significant differences were seen in motor and nonmotor outcomes between the nilotinib groups and the placebo group” (Click here to read more about this).
The NILO-PD study was a multi-centre study which also finished in 2019, and the results were published in 2020. The treatment was acceptable safety & tolerability, but data “indicate that nilotinib should not be further tested in PD“.
The researchers reported that “the low cerebrospinal fluid exposure & lack of biomarkers effect combined with the efficacy data trending in the negative direction indicate that nilotinib should not be further tested in Parkinson’s“. There was no difference in the clinical measures (change of MDS-UPDRS-3 OFF) from baseline to 6 months between the treatment groups. Cerebrospinal fluid/serum ratio of nilotinib concentration was 0.2% to 0.3% (Click here to read more about the results)

These results have left a cloud hanging over c-Abl inhibitors as a potential therapeutic class, but (and I am happy to go on the record here – giving an actual opinion!) based on the extremely low levels of nilotinib that actually got into the brain in these studies (0.2% to 0.3% of the level in serum), I don’t think we have had a proper test of the c-Abl inhibition theory in Parkinson’s yet.
And I will not be satified until a brain-penetrant c-Abl inhibitor has been tested.
Luckily, numerous brain-penetrant c-Abl inhibitors are already in clinical trials for Parkinson’s.
Chief among these is Vodobatinib (formerlu known as K0706), which is being developed by Sun Pharma Advanced Research Company (or SPARC).

The Cure Parkinson’s Trust was a supporter of the PROSEEK study.
Another c-Abl inhibitor being targeted at Parkinson’s is FB-101, which is being developed by the biotech firms 1ST Biotherapeutics.
FB-101 has now entered Phase I clinical testing in healthy volunteers (Click here to read more about that study). The study was scheduled to finish mid 2020, so we will be looking for an update about this clinical programme in 2021.
It is also interesting to note that 1stBio is collaborating with the biotech firm Neuraly to bring more c-Abl inhibitors to the clinic (Source).
In late 2020, we learned that another brain penetrant c-Abl inhibitor called Radotinib, has entered clinical trial for Parkinson’s. This agent is being developed by South Korean firm Ilyang Pharmaceutical.
Radotinib is currently in a Phase II randomized double-blind, placebo-controlled study involving 40 participants. The trial is scheduled to finish in April 2022 (Click here to read more about this trial).
And another biotech company – Inhibikase Therapeutic – has started Phase I testing their c-Abl inhibitor for Parkinson’s. This agent is called IkT-148009. The Phase I trial is being conducted in 112 healthy elderly volunteers and is scheduled to finish in March 2021 (Click here to read more about this).
In addition to c-Abl inhibitors, there are additional biological targets involved with autophagy that are being clinically explored for Parkinson’s. One example of this is a drug called RTB101 (Dactolisib), which was being developed by resTORbio Inc.
RTB101 is a TORC1 inhibitor. TORC1 is a master regulator of autophagy, and by inhibiting it, the waste disposal system of a cell is boosted. The Phase I ResTorbio clinical study is being conducted in New Zealand (Click here to read more about this trial and click here to read a SoPD post on this trial). The trial was scheduled to finish in 2020, but Restorbio announced delays due to COVID (in NZ?!? Source). We will hopefully receive an update on this study in 2021. That said, Restorbio merged with Adicet Bio in September 2020 (Source), so we will need to wait and see what Adicet have in mind for Parkinson’s.
|
Preclinical developments in autophagy research to look out for in 2021:
|
Another indirect approach to slowing the progression of Parkinson’s focuses around anti-inflammatory approaches:
The indirect approach: Anti-inflammatory approaches
When cells in your body are stressed or sick, they begin to release tiny messenger proteins which inform the rest of your body that something is wrong. When enough of these messenger proteins are released that the immune system becomes activated, it can cause inflammation.
Inflammation is a critical part of the immune system’s response to trouble. It is the body’s way of communicating to the immune system that something is wrong and activating it so that it can help deal with the situation.
By releasing the messenger proteins (called cytokines), injured/sick cells kick off a process that results in multiple types of immune cells entering the troubled area of the body and undertaking very specific tasks.

There is now a great deal of evidence to suggest that inflammation is playing a role in neurodegenerative conditions like Parkinson’s. As a result, researchers have been exploring methods of reducing the immune response in an effort to restrict the potential damage inflicted. This has led to a number of clinical trials.
In 2021, one such anti-inflammatory clinical trial will be initiated here in the UK. Researchers at Cambridge University will be testing the immunosuppressive medication, Azathioprine, in people with Parkinson’s (Click here to read more about this trial).

The South Korean biotech firm GNT Pharma is Phase I testing their anti-inflammatory drug, a mPGES-1 inhibitor called Crisdesalazine (Click here to read more about this).

This biotech firm is developing a drug called XPro1595, which targets soluble TNF. Tumor Necrosis Factor (TNF) is a potent immune signaling molecule (a cytokine) and it is intimately involved in inflammation. But Xpro1595 is different to current clinically approved TNF inhibitors, as it only neutralises soluble TNF, while not affecting trans-membrane TNF (this is an important difference).
Inmune Bio has conducted a Phase I clinical study of XPro1595 in individuals with moderate Alzheimer’s, which was scheduled to finish in December 2020 (Click here to read more about this trial). Some interim results were announced in July 2020 (Source), and we are hoping to see an update regarding this agent in 2021. If this treatment is well tolerated, it would be interesting to see this drug tested in Parkinson’s.
In 2021, I am also hoping to hear news regarding sargramostim.

In 2020, the biotech firm Alkahest initiated a Phase II clinical trial of their orally administered CCR3 inhibitor AKST4290 in 120 people with Parkinson’s (Click here to read more about this clinical trial).

Another area of inflammation-related research that is very hot at the moment is efforts to inhibit the inflammasome.
Inflammasomes are multi-protein formations, present inside of cells in your body, and they can amplify the immune response to damage or a pathogen. Recent preclinical research suggests that blocking the inflammasome can rescue models of Parkinson’s (Click here to read a SoPD post on this topic)
Researchers have also reported that the Parkinson’s-associated protein alpha synuclein can promote the activation of inflammasomes in the immune cells of the brain: the microglia.
This has given rise to the development of NLRP3 inhibitors (NLRP3 is a key protein involved in inflammasomes). The first of these inhibitors to reach the clinic is Inzomelid, which was being developed by a biotech firm called Inflazome.

Then in September 2020, it was announced that Inflazome was being acquired by the pharmaceutical company Roche:

The future development of inzomelid will also include Phase II studies for Parkinson’s, and those trials will most likely make use of an NLRP3-targeted PET imaging tracer, that Inflazome has developed with funding from the Michael J. Fox Foundation (Click here to read more about this).
|
Preclinical developments in inflammation research to look out for in 2021: Anti-inflammatory approaches:
Inflammasome targeting therapies:
|
In addition to these inflammation targeting approaches, there are other indirect approach treatments being clinically developed for slowing the progression of Parkinson’s.
The indirect approach: Gastrointestinal system
Over the last decade the role of the gut in general health has received a lot of attention with the acknowledgement that the bacteria in our gastrointestinal system is doing more than simply digesting our food.
Increasingly, we are discovering that these tiny organisms that live in symbiosis with us may also play an influential role in medical conditions such as Parkinson’s, and this has resulted in a number of clinical trials evaluating various approaches to try and adapt (or exploit) the potential of the “gut microbiota” (the communities of bacteria living in the 28 feet of our gastrointestinal tract).
One obvious way of manipulating the commuities of bacteria in our guts is to alter them with the use of antibiotics.
The MICRO-PD (“Microbiota Intervention to Change the Response of Parkinson’s Disease”) study is scheduled to finish in the second half of 2021 (Click here to read more about this study). This Phase I/II study – which is being conducted in California – involves 86 participants who are being treated with the antibiotic Rifaximin.
Another method of adjusting the composition of the flora of the gut is via dietary adjustment, and there have been studies exploring this as well.
Resistant maltodextrin is a dietary fiber that enhances gut health, and this has been clinically tested recently in Parkinson’s. In a 4-week, Phase II study involving 30 individuals, the safety and tolerability of this treatment has been assessed (Click here to read more about this study). This study was scheduled to complete in the middle of 2021, so maybe by the end of the year we will have an update about it.
There are several clinical trials evaluating probiotics – live microorganisms that are ingested.
The Symprove study here in the UK is evaluating the impact of a probiotics on 60 individuals with Parkinson’s, assessing symptoms, inflammation and the gut microbiota itself (Click here to read more about this study). That study was scheduled to complete in 2020, so we will hopefully learn about the results or have an update in 2021.
Another probiotic study called the TAP (“Treating Anxiety in Parkinson’s Disease With a Multi-Strain Probiotic”) study is focused more on symptom response to a probiotic treatment (called Ecologic® BARRIER 849) but they are also assessing clinical measures of progression (Click here to read more about this study). This study was scheduled to finish in mid 2021, but we may not learn of the results until 2022.
Not to be confused with fetal cell transplantation (which will be discuss below in component #3 below), fecal transplantation involves attempting to introduce bacteria from the gastrointestinal system of “healthy” individuals into the gastrointestinal system of people with medical conditions like Parkinson’s.
Two of these trials were scheduled to finish in 2020.
The first of them was a Phase IIa study being conducted in Israel. It involved 100 participants who have been evaluated for 6 months in a open label fashion (Click here to read more about this trial).
And the second fecal transplant study was being conducted in Houston (Texas), and wasa smaller pilot study of 12 participants being evaluated over 12 weeks following treatment with PRIM-DJ2727 (Click here to read more about this trial). This study was scheduled to be completed in late 2020, so in 2021, we will be looking out for the results or have an update of this work.
|
Preclinical developments in gastrointestinal system research to look out for in 2021: Small molecules:
Probiotics:
Gut microbiota-derived therapies:
|
While it is essential for normal health, iron does start to accumulate with age. In many cases of Parkinson’s, this process appears to be exaggerated in certain regions of the brain, and as a result researchers have tested different methods of reducing excess iron levels in models of Parkinson’s as a method of slowing down the progression of the condition (Click here to read a review on this topic).
Indirect approach: Iron chelation
Several clinical trials have recently been conducted in Parkinson’s cohorts that are exploring the use of iron chelators – this is a class of medications that reduce the levels of iron in the body.

The first of these Deferiprone studies was scheduled to finish in late 2019. This study (called the “SKY study”) recruited 140 people with early Parkinson’s and treated them twice-daily for 9 months with Deferiprone (one of 4 different doses) or a placebo drug (Click here to read more about the details of this study). We will hopefully learn the results of this trial in 2021. This international trial is being conducted by ApoPharma.
The second Deferiprone study is scheduled to finish in late 2021 (Source). This trial is called the FAIRPARK II study.
This is a larger trial than the SKY study, with 330+ participants who are being treated with either Deferiprone or a placebo treatment for 9 months (Click here to read more about this).
The Cure Parkinson’s Trust is a supporter of the FAIRPARK II study.
In 2018, Alterity Therapeutics (formerly PRANA Biotechnology) initiated a clinical trial of their iron chelator drug PBT434 in healthy individuals.
This was a Phase I study evaluating the safety, tolerability and pharmacokinetics of this treatment, after single and multiple oral dose administration (Click here to read more about that study and click here for a previous SoPD post on this topic).
|
Preclinical developments in iron chelation research to look out for in 2021:
|
Now we a going to shift gears slightly.
If one of these ‘direct’ or ‘indirect’ approaches is found to be able to slow or halt the progression of PD, the next requirement in a ‘curative therapy’ for Parkinson’s is:
COMPONENT #2. A neuroprotective agent
Once a drug or a treatment has been determined to slow down the progression of Parkinson’s, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the ‘cure’ (Cell replacement therapy – more on that below).
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions (think Alzheimer’s, Parkinson’s, Huntington’s, etc), and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards greater stratification and personalisation of disease and treatment, respectively.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinson’s: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a Glucagon like peptide-1 receptor (or GLP-1R) agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinson’s.
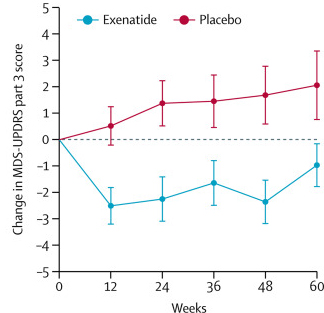
After multiple studies suggested neuroprotective properties in models of Parkinson’s, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinson’s motor features over the course of the 48 week trial (Click here and here to read previous SoPD posts about this).
The researchers found a statistically significant difference in the motor scores of the exenatide-treated group verses the placebo group (p=0·0318). As the placebo group continued to have an increasing (worsening) motor score over time, the exenatide-treated group demonstrated improvements, which remarkably remained after the treatment had been stopped for 3 months (weeks 48-60 on the graph below).
Reduction in motor scores in Exenatide group. Source: Lancet
Brain imaging (DaTScan) also suggested a trend towards reduced rate of decline in the exenatide-treated group when compared with the placebo group. Interestingly, the researchers found no significant differences between the exenatide and placebo groups in scores of cognitive ability or depression – suggesting that the positive effect of exenatide may be specific to the dopamine or motor regions of the brain.
|
NOTE: It should be made very clear here that we are still not sure if the effect of exenatide is “neuroprotective” or perhaps simply symptomatic. This needs to be carefully determined in further clinical research. |
This and other results have led to a number of research groups and biotech companies developing GLP-1R agonists for Parkinson’s.
In late 2019, we saw the initition of a Phase III clinical trial for Bydureon/Exenatide.
The Cure Parkinson’s Trust is a supporter of the Exenatide III study.
Given the Phase II results, there are high expectations for the Phase III trial which need to be carefully managed.
While it will be good to see this trial start, it is important to remember that not everyone in the Phase II trial treated with exenatide responded to the drug – that is to say, not everyone taking the treatment experienced benefits. Thus, an important part of this new trial will be trying to identify characteristics of those who do respond compared to those who do not – which could aid in future administration of the drug (if it is eventually approved).
The Phase III trial will involved 200 participants being treated for 2 years and is scheduled to complete in 2024 (Click here to read more about this trial and click here to see the trial webpage).
There is also a clinical trial evaluating exenatide in people with recently diagnosed Parkinson’s being initiated in Sweden. It is a Phase II study that will recruit 60 participants and follow them for 18 months (Click here to read more about this study).
The University of Florida also has a small clinical trial evaluating exenatide in people with Parkinson’s. This study is scheduled to finish in 2021 (Click here to read more about this study).
In addition to Exenatide, there are additional ongoing clinical trials for other GLP-1R agonists, for example the Lixisenatide trial in France (Click here and here for more information on this study), and the Liraglutide trial in California (Click here and here to read more about this study).

In addition, there is a clinical trial of a new formulation of Exenatide called Semaglutide in Parkinson’s. This study is being conducted by researchers in Norway who are accessing the drug via the Danish biotech firm Novo Nordisk.
This Phase II study will involve 120 participants, who will take either Semaglutide (or placebo) for 24 months in a double blind fashion, before a further 24 months of open label assessments. The study is scheduled to finish in 2024 (Click here to read more about this study).
Biotech firm Neuraly initiated a Phase II trial of their GLP-1R agonist NLY01 in February 2020. That study is a Phase II 36-week, placebo controlled, double-blind trial involving 240 participants, and it is scheduled to complete in August 2022 (Click here to learn more about that study).
Another new entrant to the GLP-1R agonist group is Peptron.
This South Korean biotech firm have also conducted Phase I safety testing of their GLP-1R agonist (called PT320 – click here to read more about that trial). In 2020, Peptron initiated a Phase IIa study to evaluate the efficacy and safety of 48 weeks treatment with PT320 in 100 individuals with early Parkinson’s. This study is scheduled to complete in late 2021 (Click here to read more about that trial).
|
Preclinical developments in GLP-1R agonists research to look out for in 2021:
|
Another form of ‘neuroprotective’ treatment that has been tested in Parkinson’s for a long time is neurotrophic factors.
Neuroprotective approach: Neurotrophic factors
Neurotrophic factors are supportive/nurturing proteins that the brain produces naturally. They help to keep cells alive by activating and stimulating specific biological pathways. There are different types of neurotrophic factors which affect different types of neurons in different ways (one of my pet peeves is repetition of a word in a single sentence!).

Glial cell-derived neurotrophic factor (GDNF)
One neurotrophic factor that has a particularly robust effect on dopamine neurons (the type of neurons most severely affected by Parkinson’s) is GDNF (or Glial cell-derived neurotrophic factor).
In 2019, we saw the publication of the Bristol Phase II GDNF clinical trial results. GDNF is a neurotrophic factor that has had a long roller coaster-like history with Parkinson’s. The results of the Phase II trial indicate that there was no significant improvement in those individuals being treated with GDNF compared to those on the control/placebo treatment (as measured by clinical rating scales – click here to read a SoPD post about these results).
The Cure Parkinson’s Trust was a supporter of the Bristol GDNF study.
Despite the set back with this GDNF trial, there is another GDNF trial that is investigating a gene therapy form of GDNF. A biotech firm called Brain Neurotherapy Bio published the results of a Phase I clinical trial assessing their AAV-based GDNF gene therapy treatment in people with Parkinson’s in 2019, and in 2020 this company was acquired by the pharmaceutical company Bayer (Click here to read a SoPD post on this topic).
The company is conducting a follow-up open-label safety study of their approach which is started in 2020 (Click here to read more about this trial). This trial will provide further case for support to conduct a much larger clinical trial. The initial results of this study are scheduled to be announced in late 2022.
Another neurotrophic factor that is currently in clinical trial is CDNF:
Cerebral dopamine neurotrophic factor (or CDNF).
This protein is different to GDNF, but has also been shown to have beneficial effects on dopamine neurons (Click here to read a good review on this topic).

CDNF is being developed as a therapy for Parkinson’s by the biotech firm, Herantis.
The results of their Phase I/II study evaluating the safety and tolerability of CDNF in people with Parkinson’s were announced in 2020 (Click here to learn more about this and click here to read a SoPD post about this). Alternatively, you can watch this video:
Similar to the Bristol Phase II GDNF study (mentioned above), the drug was injected directly into the brain using an implanted canular system. One-third of the participants received monthly infusions of placebo and two-thirds of the participants received monthly infusions of either mid- or high-doses of CDNF for 6 months (Click here to read more about this trial).
The results indicate that the treatment was safe and well tolerated, but the company has decided “to pursue more patient-friendly modes of delivery that do not require the need for a surgical device”, rather than pursue a larger Phase II study (Source). Given the invasive nature of this procedure, this is probably a wise move. And the company has already announced developments in this new direction by forming a collaboration with Nanoform to develop a nasal drug delivery approach to get CDNF and xCDNF (a smaller version of CDNF that Herantis is developing) into the brain (Source).
|
Preclinical developments in neurotrophic research to look out for in 2021:
|
In addition to the research being conducted on GLP-1 agonists and neurotrophic factors, there are additional neuroprotective approaches being explored.
Neuroprotective approach: Additional
A number of research groups are exploring the idea of using plasma – the portion of blood that doesn’t contain cells – from young organisms to improve function in older creatures. The hypothesis is that this “young blood” may contain beneficial properties for older organisms.
Not quite Count Dracula, but there has been a lot of research exploring this idea (Click here to read a SoPD post on this topic). There have also been a number of clinical trials investigating this as an approach, including for Parkinson’s.
In 2020, we saw the results of the Stanford Parkinson’s Disease Plasma Study (SPDP) published (Click here to read a SoPD post about those results).

The results suggest that not only was the treatment safe and well tolerated, but elevated baseline measures of inflammation (such as blood levels of tumor necrosis factor‐α) were decreased 4 weeks after the infusions ended.
The study was too small and short to provide any meaningful measures of efficacy, but the investigators conducting the study suggested that the results “warrant further therapeutic investigations in PD”.
In addition to this study in Stanford, a clinical trial of a product derived from young blood plasma (called GRF6021) was scheduled to finish in 2020. This trial is being conducted by a biotech firm named Alkahest.
The study was a randomised, double-blind, placebo-controlled Phase II study in 90 people with Parkinson’s and cognitive impairment. It was assessing the safety and tolerability of GRF6021 over a period of 7 months. The treatment (or placebo) was administered by intravenous infusion for 5 consecutive days at Week 1 and Week 13 of the study (Click here to read more about the details of this clinical study). We hope to hear an update regarding this study in 2021.
In 2020, the results of a clinical trial of a drug called ANAVEX2-73 were announced. The drug is a Sigma-1 agonist (Click here to read a review on Sigma-1 biology) and it is being developed by the biotech company Anavex Life Sciences.
This was a Phase 2, double-blind, placebo-controlled study evaluating the safety, tolerability, and efficacy of ANAVEX2-73 in 120 people with Parkinson’s with dementia. The participants were treated for 14 weeks and the assessments were primarily focused on cognitive measures (Click here to read more about the details of this trial).
The results demonstrated a “clinically meaningful, dose-dependent, and statistically significant improvements in the Cognitive Drug Research (CDR) computerized assessment system analysis” (Click here to read more about the results). A 48 week open label extension study for the participants of this study is now being conducted (Click here to read more about that study). It will be interesting to learn how this extension study is going in 2021.
A large Phase III clinical trial of the effects of Lingzhi (also known as Ganoderma or reishi in Japan) on disease progression in 288 people with recently diagnosed, untreated Parkinson’s was scheduled to finish in December 2020 (Click here to read more about this study). Limited preclinical research has been published in the west with this extract of an oriental fungus in models of Parkinson’s (for examples, click here and here), but the results of this clinical trial could be something else to keep an eye out for in 2021.
|
Preclinical developments in neuroprotective additional approaches research to look out for in 2021:
|
Phew, long post.
If you have read this far in just one sitting, I owe you a beer.
And now, we move on to 2021 clinical trials involving the third component of any ‘curative therapy’ for Parkinson’s:
COMPONENT #3. Some form of restorative therapy
Once the condition has been slowed/halted (component #1) and a neuroprotective/nurturing environment is in place to protect the remaining cells (component #2), a curative treatment for Parkinson’s will require replacing some of the cells that have been lost.
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain. These cells are critical for normal motor function – without them, movement becomes very inhibited, resulting in the slowness of movement and rigidity symptoms associated with the condition.
And until we have developed methods that can identify Parkinson’s long before the motor features appear (which would require only a disease halting treatment), some form of cell replacement therapy is required to introduce new cells to take up lost function.
Cell transplantation currently represents the most straight forward method of cell replacement therapy.
Cell Transplantation
Traditionally, the cell transplantation procedure for Parkinson’s has involved multiple injections of developing dopamine neurons being made into an area of the brain called the putamen (which is where much of the dopamine naturally produced in the brain is actually released). These multiple sites allow for the transplanted cells to produce dopamine in the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).
Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis – of the brains of individuals who have previously received transplants of dopamine neurons and then subsequently died from natural causes – has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain. In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells (stained in brown). A magnified image of individual dopamine producing neurons can be seen in panel B:
Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine.
As you can see in the images below (a bird’s eye view of the brain from above), there is less dopamine being processed (indicated in red) in the putamen on each side of the Parkinsonian brain on the left than the brain on the right (several years after bi-lateral – both sides of the brain – transplants):
Brain imaging of dopamine processing before and after transplantation. Source: NIH
The old fashioned approach to cell transplantation involved dissecting out the region of the developing dopamine neurons from multiple donor embryos (these are referred to as fetal transplants – different to the fecal transplants discussed further above). The tissue was broken into small pieces that could be passed through a tiny syringe, and this was injected into the brain of a person with Parkinson’s.
The people receiving this sort of transplant would require ‘immunosuppression treatment’ for long periods of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
|
SMALL SIDE NOTE HERE: It is very important for all readers of this post to appreciate that cell transplantation for Parkinson’s is still extremely experimental. Anyone declaring otherwise (or trying selling a procedure based on this approach) should not be trusted. There are no cell therapy approaches for Parkinson’s that have been approved by any medical regulators. While I appreciate the desperate desire of the Parkinson’s community to treat this condition ‘by any means possible’, bad or poor outcomes for this technology could have serious consequences for the individuals receiving the procedure and negative ramifications for all future research in the stem cell transplantation area. |
There is an ongoing European clinical trial called Transeuro which is using the fetal cell approach for Parkinson’s.
The Cure Parkinson’s Trust is a supporter of the TRANSEURO study.
This trial is an open label study, involving 13 subjects. This study is scheduled to finish in 2021 (Click here to read more about this).
There is also a South Korean clinical trial being conducted at Bundang CHA Hospital, assessing fetal dopamine neurons in 15 participants and evaluating them over 5 years. That study is scheduled to finish in 2022 (Click here to read more about this).
In addition to these two fetal cell transplantation studies, there are currently 4 ongoing stem cell-based clinical trials for Parkinson’s.
The first of those clinical trials is the ‘induced pluripotent stem’ (or IPS) cell study being conducted in Kyoto, Japan (Click here to read a SoPD post on this topic).
This ongoing cell transplantation trial is being conducted by researchers at the Center for iPS Cell Research and Application (or CiRA).

Information provided by Kyoto University (Click here for the study website) and the Japanese media has indicated that this Phase I/II clinical trial aims to investigate the safety and efficacy of transplanting human IPS cell-derived dopaminergic progenitors into the brains of people with Parkinson’s (what stage of PD has not been disclosed). The study will involve just 7 participants, who are all Japanese individuals with Parkinson’s. The participants in the study will be followed and assessed for 2 years post transplantation
According to the CIRA 2018 annual report, the researchers began enrolling patients on 1st August and the first patient was transplanted in October (2018 – Click here to read the annual review). The investigators stated that the gentleman who was transplanted will be monitored for 2 years…which is October 2020, so hopefully in 2021 we will receive an update as to how this trial is going
And perhaps we will receive an indication of how this programme will evolve. In September 2020, Kyoto University announced plans to “make induced pluripotent stem cells, known as iPS cells, promptly and widely available at lower cost” in 2021 (Source). The Japanese have big plans for stem cells.
In 2018, a second stem cell-based cell transplantation clinical trial was initiated by a research group from China led by Professor Qi Zhou, a stem-cell specialist at the Chinese Academy of Sciences Institute of Zoology.
This study is taking place at The First Affiliated Hospital of Zhengzhou University in Henan province, and involves 10 participants being injected with neuronal-precursor cells derived from embryonic stem cells (Click here to read more about this trial). The study is scheduled to finish in late 2020 (Click here to read a previous SoPD post on this topic).
The third trial is being conducted by Allife Medical Science and Technology Co.

And the fourth on-going stem cell-based cell transplantation clinical trial is being conducted in Melbourne (Australia), by an American company called International Stem Cell Corporation (ISCO).
This study involves the use of a different type of stem cell to produce dopamine neurons for transplantion in the Parkinsonian brain (Click here and here to read previous posts about this).
Specifically, the researchers will be transplanting human parthenogenetic stem cells-derived neural stem cells (hpNSC). These hpNSCs come from an unfertilized egg – that is to say, no sperm cell is involved. The female egg cell is chemically encouraged to start dividing and then it becoming a collection of cells that is called a blastocyst, which ultimately go on to contain embryonic stem cell-like cells.
This process is called ‘Parthenogenesis’, and it’s not actually as crazy as it sounds as it occurs naturally in some plants and animals (Click here to read more about this). Proponents of the parthenogenic approach suggest that this is a more ethical way of generating embryonic cells as it does not result in the destruction of a viable organism. The stem cells derived from this process can be grown into neuronal cells before being transplanted into brain.
The ISCO trial involves 12 participants who have been evaluated over 12 months in this evaluation of safety and tolerability. The study was scheduled to finish in 2020 (Click here to read more about this trial), so we will hopefully have an update in 2021.
As you can see there should be a great deal of data in 2021 regarding the cell transplantation clinical trials that have been conducted over the last few years.
We should also be seeing the start of new clinical trials as well.
On the 7th January 2021 a biotech company called BlueRock Therapeutics announced that they have received the green light from the US FDA to start their clinical trial studies (Source).

The first clinical trial to be initiated by the company will be a Phase I study is to assess the safety and tolerability of their DA01 cell transplantation at one-year post-transplant. This will probably be a small study, involving less than 10 individuals – though the details have still not been announced.
Another biotech company that will be seeking to start clinical testing in 2021 is Aspen Neuroscience.

In April 2020, Aspen announced that they had raised US$70 million in Series A funding to help progress development of their programme (Click here to read a SoPD post on this topic). Hopefully they too will get the green light from the US FDA in 2021 to start clinical testing.
|
Preclinical developments in cell transplantation research to look out for in 2021: In addition to these active (and near) clinical programmes, there are a number of preclinical efforts underway
There are also new biotech companies popping up that are exploring the concept of directly converting cells in the brain into dopamine neurons as a therapy for Parkinson’s. These include NeuExcell Therapeutics who have an active research programme for Parkinson’s (source). |
Ok, that’s it.
I think we are done.
It’s not everything, but it will certainly give you a flavour of where things currently stand and what to look out for in 2021, regarding clinical trials for disease modification in Parkinson’s.
One complaint regarding this particular post each year is that all of these trials are focused on drugs/gene therapies/etc. It is a fair comment, but broadening the scope of this post would be the death of me I suspect. For those interested in exercise, there are a lot of clinical trials that have just registered or recruiting (96 at the time of writing) which could be explored (Click here to read more about these).
SUMMARY: The main take-aways
I will be brief here, but there are a couple of minor thoughts for your consideration:
- Treatments targeting the Parkinson’s-associated proteins/pathways alpha synuclein, GBA and LRRK2 are all well covered. We have a lot of activity in all three of these areas. I suspect that until we see any signals of efficacy from therapies targeting these proteins, we are probably best to leave the early parties to their work and focus our attention on developing experimental treatments targeting novel pathways.
- It is really positive to see novel targets and mechanisms of action being explored and brought forward to the clinic. The genetics work of the early 21st century has highlighted a number of biological pathways that appear to be involved in different aspects of Parkinson’s, and early basic research exploring these areas is now starting to bear fruit. This is really encouraging.
- The good news from this post is that there is a lot of research focused on slowing the progression of Parkinson’s, but restorative therapies are still lacking. One of the themes on the SoPD in 2020 was exploring novel restorative approaches to Parkinson’s that didn’t involve cell transplantion. Most of the methods discussed were rather ‘blue sky’, but it would be very encouraging if in the next “Road ahead” post we could add an additional treatment category under component #3 some form of restorative therapy.
I would be remise if I did not make one last statement regarding the need to check expectations. While this mammoth list of clinical trials bodes well for the future of Parkinson’s treatments, there are no guarantees that any of them are actually going to work. In addition, we must remember that a clinical trial is supposed to be an unbiased evaluation of a treatment. If we enter a clinical trial with any expectation (as a participant or observer), we are by definition biased. We should approach all of these trials with no expectations, and evaluate the outcomes not based on success or failure, but on what we have learnt (and yes, I appreciate that is easy to say for one who is not affected by the condition).
The amount of research being conducted is a very positive sign, however, and it suggests that 2021 will be an extremely productive year of Parkinson’s research. And there is certainly going to be a lot of clinical trial results being announced in 2021!
But now it’s time to give the fingers (and brain) a wee rest.

Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The author of this post is an employee of the Cure Parkinson’s Trust, so he might be a little bit biased in his views on trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Weknowyourdreams